The Meda Pharma laboratory is recalling several lots of Epipen autoinjector pens around the world. A production incident would be involved.

The National Medicines Safety Agency (ANSM) has launched a recall procedure for lots of Epipen autoinjector pens due to a quality defect. This faulty workmanship “results in a potential malfunction of the injection system and an inability for the patient to administer this drug”, explains the health agency. To date, no case has been reported in the country.
Epipen is an adrenaline drug used for the emergency treatment of anaphylactic shock caused by allergy. This can be related to food, medicine or the bite of an insect. Anaphylactic shock can also be caused by exercise.
Global recall
The Meda Pharma laboratory markets two strengths in France: Epipen 0.15mg / 0.3ml used in children from 15 to 30 kg and Epipen 0.30 / 0.3ml used in adults or in children and adolescent over 30 kg.
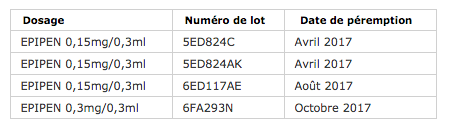
The defect was observed for 4 batches, including 3 for the 0.30 / 0.3ml dosage (see table below). This imperfection is linked to “a one-off production incident” which impacted several batches sold around the world, the ANSM explained in its press release. Also, the laboratory is carrying out a worldwide recall of these affected products.
A toll-free number available
The ANSM asks all patients with this device to check whether it is on the list of recalled products. To do this, they must check the lot number indicated on the Epipen pen as well as the box. “If this is the case, patients will have to come to their pharmacy with the pens. Pharmacists will then proceed to the exchange with other Epipen pens made available free of charge in this context, by the MEDA Pharma laboratory “, explained the agency, recalling in passing that” patients must always be in possession of two pre-filled pens as a precaution ”. If in doubt, le toll free number 0800 849 033 was set up by the laboratory.
At the same time in Canada, the Mylan laboratory is also recalling lots of Epipen pen. These are the EpiPen 0.3 mg auto-injectors (5GU763) expiring May 2017, and a batch of EpiPen Jr 0.15 mg auto-injectors (5GR765) expiring in March 2017.
.















