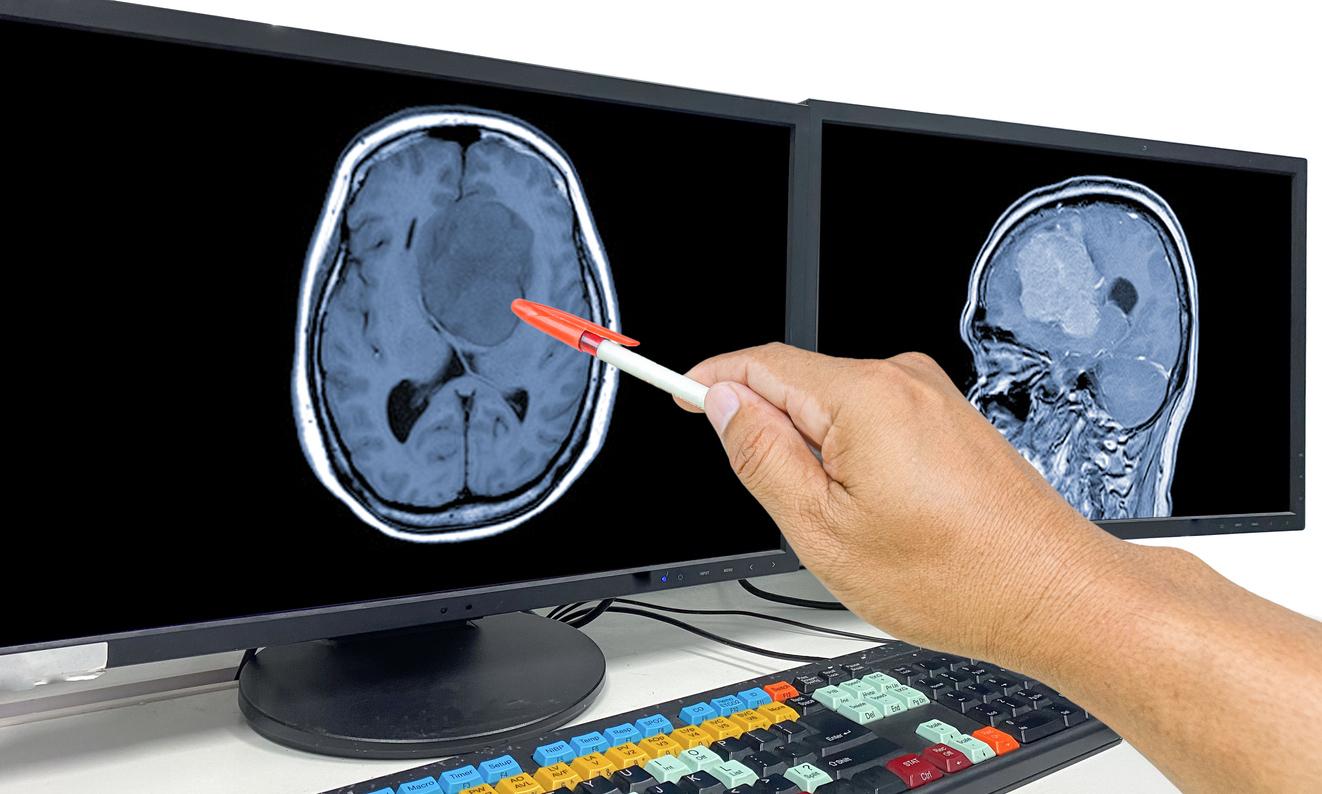
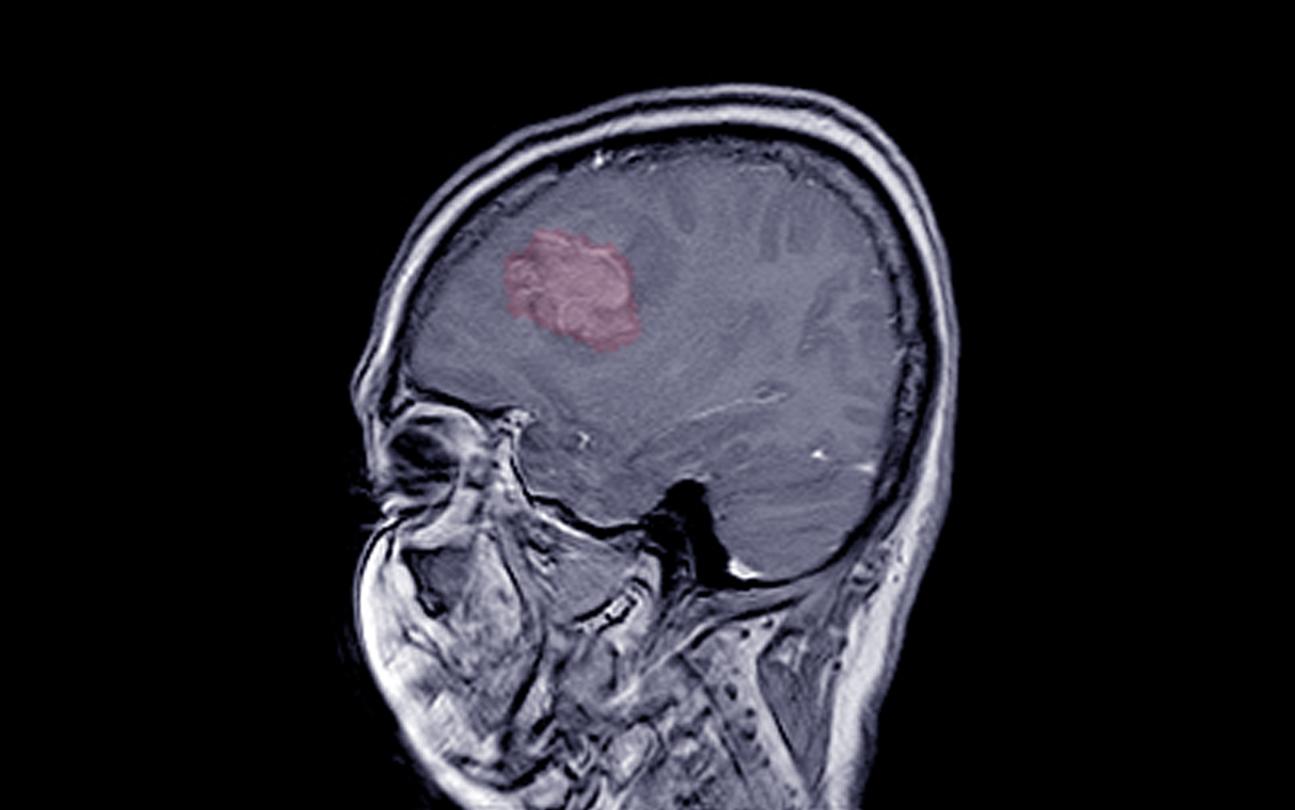
The National Medicines Agency warns against three treatments, prescribed to many women, which would promote the occurrence of brain tumors.

- Taking three progestins, Surgestone, Depo Provera and Colprone, is associated with an increased risk of meningioma.
- Their use for more than a year multiplies the risk of brain tumors, respectively by 4.1, 2.7 and 5.6.
- No increased risk of meningioma was observed with hormonal IUDs.
In the event of endometriosis, fibroids, particularly long and/or heavy periods and cycle disorders, doctors prescribe progestins to women. These drugs are also used in hormone replacement therapy (including for menopause) and in obstetrics (sterility due to luteal insufficiency, repeated abortions). Problem: some progestins could increase the risk of meningioma (i.e. an almost always benign tumor that develops from the meninges), according to the National Agency for the Safety of Medicines and Health Products (Ansm).
Meningioma: taking three progestins for more than a year is associated with an “excess risk”
To reach this conclusion, EPI-PHARE conducted a new pharmacoepidemiology study to assess the risk of operated intracranial meningioma in patients linked to the use of progestins (progesterone, medrogestone, medroxyprogesterone, dydrogesterone, promegestone, dienogest ). The risk of meningioma linked to hormonal IUDs was also analyzed. As part of this work, more than 18,000 women operated on for a meningioma and more than 90,000 “control” women were recruited.
“Prolonged use of promegestone, medrogestone or medroxyprogesterone acetate is associated with an increased risk of meningioma. This is increased when the duration of use of these drugs at the dosage authorized by the marketing authorization market exceeds a year, as is the case with chlormadinone, nomegestrol and cyproterone acetates”, can we read in the Ansm press release.
No increased risk of meningioma with hormonal IUDs
On the other hand, the results with hormonal IUDs, with levonorgestrel 13.5 and 52 mg, do not show an increased risk of meningioma. Another finding: exposure to progesterone (oral, intravaginal and cutaneous) and to dydrogesterone was not significantly associated with an increased risk of intracranial meningioma surgery. “Due to the absence of reimbursement for dienogest alone during the study period and the low prevalence of dienogest associated with estradiol, this study cannot conclude on the presence or absence of risk of meningioma associated with dienogest. “, the agency said.





-1730888646.jpg)