Women seek compensation for damages suffered from the Administrative Court for not having been informed of the risks of meningiomas, meningeal tumors, linked to taking Androcur, a medication prescribed for years for acne and hair loss. or as a contraceptive.
- Androcur is a medication containing a progesterone derivative and having an anti-hormonal action which has been prescribed to thousands of women since the 1980s to treat hair loss, acne or even serve as a contraceptive.
- In 2011, the risk of meningioma was mentioned in the Androcur package insert and, today, it is only prescribed to treat prostate cancer and hirsutism in women.
- More than 450 patients are now seeking compensation for damages suffered from the Administrative Court and want the State to compensate them.
“Androcur was prescribed to me by my gynecologist from 2005 as a contraceptive but mainly to treat hormonal acne, explains Émilie, 40, who took this medication for 14 years, to the Association Meningiomas due to Cyproterone Acetate, help for victims and consideration of other molecules (AMAVEA). In June 2019, I received a letter from the National Medicines Safety Agency (ANSM) inviting me to contact my prescribing doctor and recommending an MRI.”On August 13 of the same year, the diagnosis fell: “a huge protean mass in the frontal lobe measuring 6.3 cm and a smaller one, measuring 2.3 cm, at the back of the skull.”
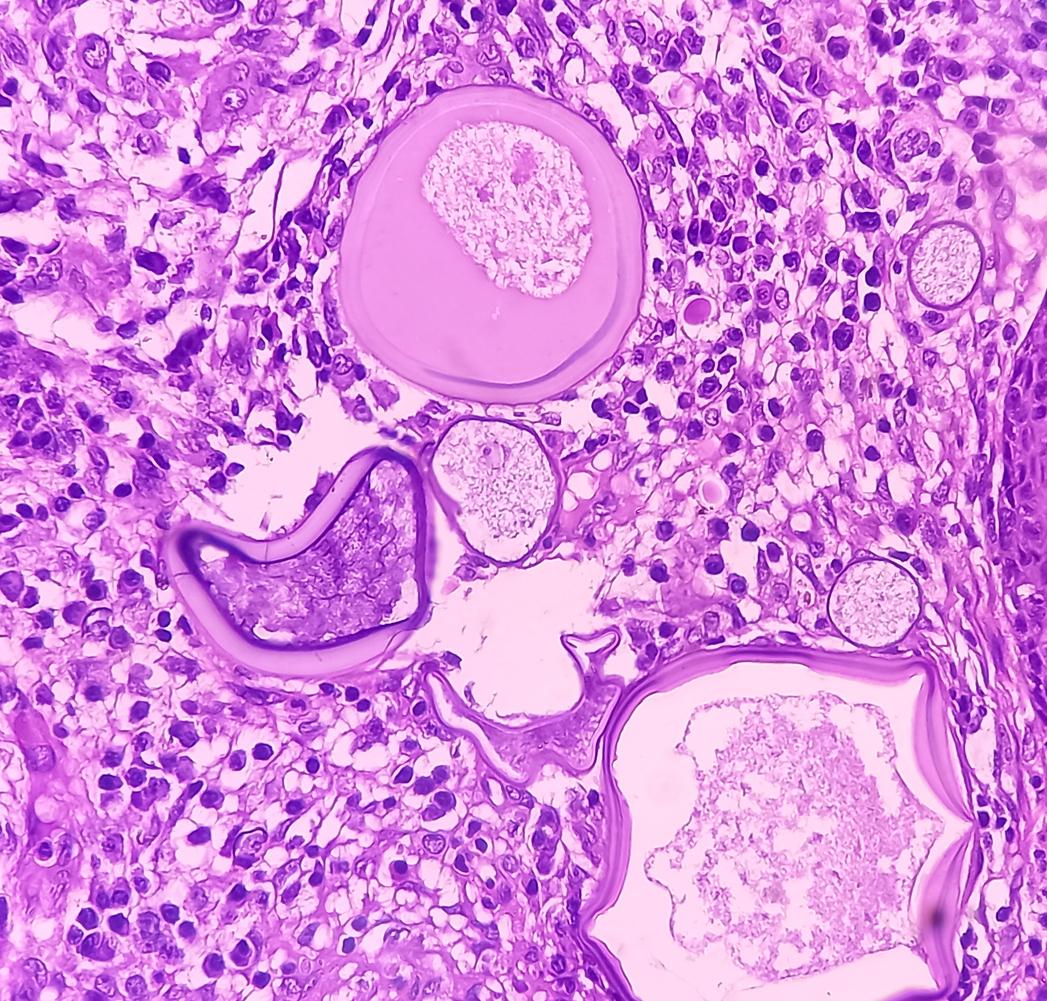
Proven risks of meningiomas
Like Émilie, many women have developed meningiomas, meningeal tumors, after taking Androcur. This medication containing a progesterone derivative and having an anti-hormonal action has been prescribed to thousands of patients. since the 1980s to treat hair loss, acne, hirsutism or even serve as a contraceptive. Problem: it is only 2011 that the risk of meningioma was mentioned in the Androcur leaflet.
To better quantify this risk, Health Insurance carried out a study. THE results were published in 2018: the risk of meningiomas linked to prolonged use of cyproterone acetate (the active substance in Androcur) at high doses (greater than or equal to 25 mg/day) was multiplied by 7 for women who took it over a long period (more than 6 months) and by 20 after 5 years of treatment.
More than 450 patients want the State to compensate them
In March 2024, two requests were filed with the Montreuil Administrative Court. “The fault that we attribute to the State is as follows: not having correctly disseminated information relating to the health risk posed by taking Androcur to doctors and patients, explains Maître Charles Joseph-Oudin, lawyer for the association, to The Dispatch. Consequence: the latter respectively continued to prescribe and consume the molecule.“
According to the words of Emmanuelle Huet-Mignaton, president of the association helping victims of Androcur (AMAVEA), to La Dépêche: “Between 2007 and 2023, 4,000 women will have had meningioma surgery because of a progestin.“
It is now up to the courts to assess the more than 450 patient files. Today, Androcur is only used, according to the Vidalto treat prostate cancer and hirsutism in women, “when excessive hair growth has a serious impact on emotional and social life.”