French researchers from GIS Epi-Phare have confirmed the existence of an increased risk of meningioma in women linked to prolonged intake of these progestins: Colprone, Depo Provera and Surgestone.
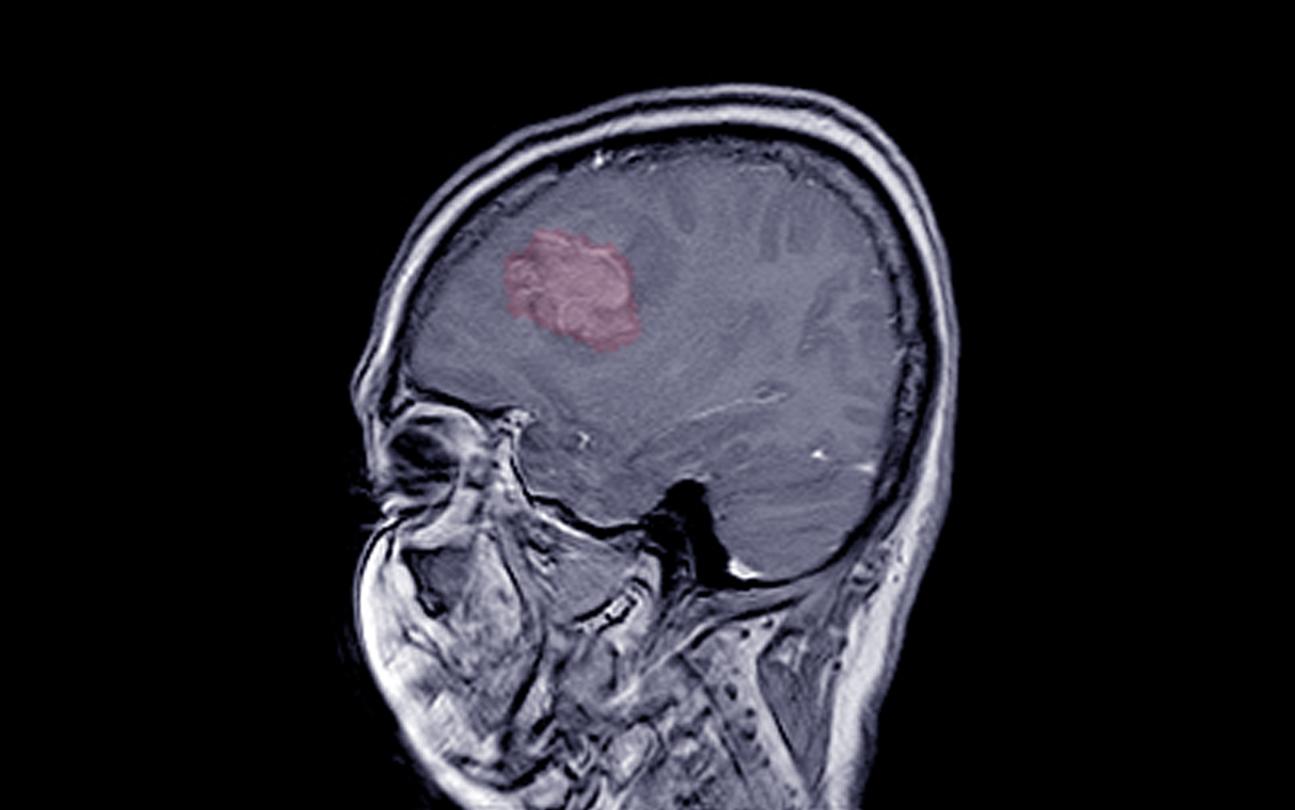
- A French study confirms a link between prolonged taking of the progestins Colprone, Depo Provera and Surgestone and an increased risk of meningioma.
- The risk of developing this type of brain tumor increases up to 5.6 times depending on the medication.
- Patients should have an MRI if there are signs of a brain tumor and after one year of taking treatment.
While previous work had already highlighted an increased risk of a brain tumor called meningioma after prolonged and high-dose use of progestogen treatments Androcur, Lutéran and Lutenyl, a study by GIS Epi-Phare reveals a similar risk with 3 other progestins: Colprone, Depo Provera and Surgestone.
The study was published in the journal British Medical Journal on March 27, 2024.
Progestins: risks of meningioma up to 5.6 times higher
French researchers took the files of 18,061 women aged 45 to 74 (on average 58 years) and operated on for a meningioma between 2009 and 2018 in France as well as those of 90,305 control women. Analyzes revealed that prolonged use (one year or more) of Colprone (treatment for premenopause, endometriosis, bleeding; active substance: medrogestone) is associated with a 3.5 times higher risk of meningioma. Women who have taken the injectable contraceptive Depo Provera (medroxyprogesterone acetate) over a long period of time have a 5.6 times greater risk of developing a brain tumor. Patients who were exposed for a year or more to promegestone (Surgestone), before the end of its marketing in 2020 in France, have a risk multiplied by 2.
For the Epi-Phare team, there is no doubt: their publication comes “once again confirm an effect of progestins on the risk of intracranial meningioma”.
What about levonorgestrel intrauterine devices like the Mirena, Donasert, Kyleena and Jaydess hormonal IUDs? The results are “very reassuring and in favor of the absence of risk of meningioma”.
Colprone, Depo Provera, Surgestone: an MRI recommended in case of signs of tumor
The 3 treatments had already been in the sights of the health authorities for several months. In the summer of 2023, the ANSM indicated that the recommendations made to patients having taken Lutéran or Lutényl should be the same for those using medrogestone (Colprone) or medroxyprogesterone acetate (Depo Provera) “to the extent that meningioma risk levels are comparable.”
Women using these products are advised to perform a brain MRI if there are signs suggestive of a brain tumor as well as after one year of treatment, 5 years then every two years. Patients who have taken Surgestone for more than a year should also consult if they experience symptoms. The signs to look out for are:
- headaches or diffuse or localized headaches: the pain worsens despite taking analgesics;
- vision, sensitivity, speech or sight problems;
- behavioral or memory problems;
- weakness in the arms or legs, or even paralysis;
- loss of balance and dizziness, loss of hearing.