Euthyrox, the old formula of Levothyrox, will be marketed again on a temporary basis, up to 90,000 treatments per quarter.

Following on from the announcement made on Tuesday by the Minister of Health, the National Medicines Safety Agency (ANSM) confirmed that the old formula of Levothyrox, now called Euthyrox, will be available from October 2 in pharmacy.
Its sale will nevertheless be limited. The health agency specifies that its availability will be temporary, and reduced to 90,000 treatments sold in quarterly packaging. Only people who cannot support other treatments will have access to it.
“It should be prescribed exclusively as a last resort to patients, in a limited number, who encounter lasting adverse effects with other drugs”, specifies the ANSM in a press release.
Alternatives available
To offer more alternatives, the ANSM also announces that the French market will open up to competition. “From mid-October, Merck will no longer have a monopoly on levothyroxine (the active ingredient in Levothyrox, editor’s note) on the French market, ”said Agnès Buzyn on RMC this Tuesday.
A drug produced by the Sanofi laboratory, L-Thyroxin Henning, will indeed also be available. It has already been sold in Germany for several years. Boxes distributed in France will be imported, and a notice in French will be provided by the pharmacist. “Subsequently, an application for marketing authorization for the laboratory will allow sustainable marketing”, specifies the ANSM.
Finally, the prescription will be open for a third formulation, L-Thyroxine from Serb laboratories. It is a drinkable solution in drops, in principle reserved for children, but which had already been prescribed to certain patients suffering from side effects with the new formula of Levothyrox. Its use will nevertheless be reserved for children under 8 years old, people suffering from swallowing disorders, and all patients who have already been prescribed this specialty before August 31, 2017.
Others to come
All these formulations will be available in several strengths, between 25 and 200 mg for Levothyrox and Euthyrox, between 25 and 150 mg for L-Thyroxin Henning, and at 150 mg / mL for drinkable L-Thyroxine Serb.
The ANSM reminds that patients who do not encounter a problem and are stabilized with the new formula of Levothyrox must not change treatment, and that any modification or discontinuation of therapy must be considered with a doctor.
For the others, in order to increase the therapeutic offer and satisfy as many patients as possible, other drugs could be marketed in the coming months.
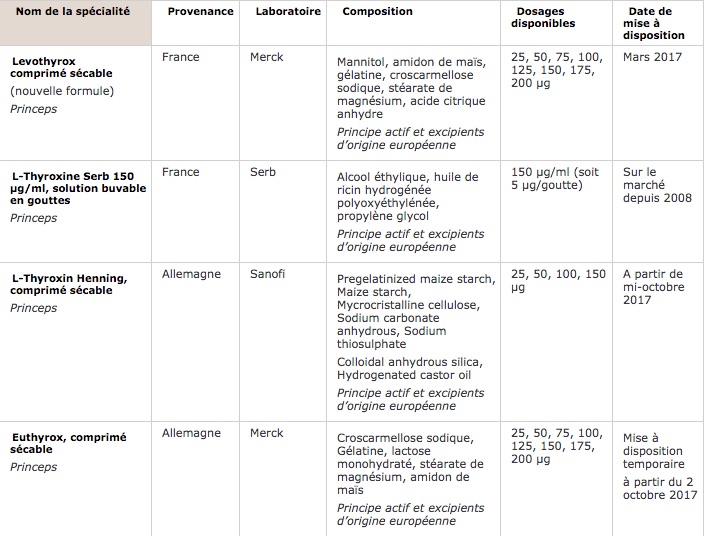
Levothyroxine-based specialties available (Source: ANSM)
.















