An ANSM analysis reveals 8 contamination incidents per year with endoscopes. One in five is due to the device, the others are due to faults in practice.

As part of its medical device surveillance and health product safety information missions, the National Medicines Safety Agency (ANSM) published on Tuesday a review of medical device vigilance reports relating to endoscopes, for the period from 2010 to 2013. These widely used optical devices make it possible to explore a natural body cavity for the purpose of diagnosis, intervention or sampling (biopsy).
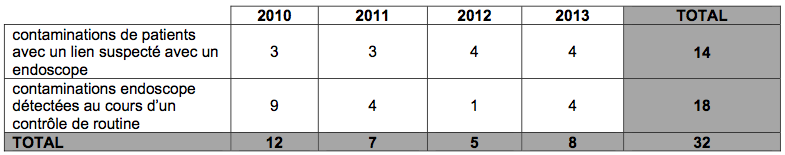
8 contamination incidents per year
Analysis of the reports reveals, on average each year, 8 contamination incidents. In more detail, we are talking here about the contamination of patients due to a suspected link with an endoscope or the contamination of endoscopes detected during a routine microbiological test.
In total, 22% find their cause in the device itself. These relate in particular to a model, the “TJF 145” of the company Olympus for which the agency issued recommendations in December 2010. The other incidents are due to poor practices (maintenance, cleaning, disinfection, etc.).
The most frequent incidents, excluding contamination
In addition, the ANSM recorded 125 reports of incidents concerning the field offlexible endoscopy between 2010 and 2013, excluding contamination. “The range of devices affected by these reports is quite wide and the causes identified are very diverse,” says the Agency.
The most frequent incidents of this period concern swabs (type of brush with handle, often cylindrical head) used for cleaning endoscopes (22 incidents).
“This is in particular due to a problem with the breaking of these swabs observed in 2013 for a manufacturer who modified a raw material used in the manufacture of its products and which caused a large number of breaks during use”, explain the authors. of the balance sheet.
The second cause of these reports is the malfunction of the endoscope itself. The ANSM has thus listed 17 incidents but which are spread over 10 categories of different devices and 17 different models.
-50% of reports on washer-disinfectors
Finally, Endoscope Washer-Disinfectors (LDE), which resemble large washing machines, were the subject of 53 medical device vigilance declarations during this period (2010-2013), with very variable types of causes. The most frequently reported incidents are: alarm triggering faults (17%), internal circuit contamination (13%), or endoscope damage (11.3%).
For these devices, however, the good news is that the Agency has noted a 50% reduction in medical device vigilance reports.
.















