The National Medicines Safety Agency submits its report on Depakine on August 24. 10,000 pregnant women have taken this teratogenic drug.
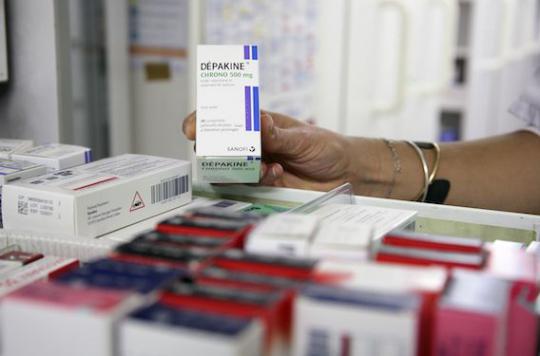
For more than a year, tensions and recriminations have been accumulating around the Dépakine file. This anticonvulsant, intended for people with epilepsy or bipolar disorder, should not be prescribed to pregnant women. It causes physical deformities and neurobehavioural disorders in the exposed baby in utero. But the prescriptions continue despite the risks, now proven. On August 24, the National Medicines Safety Agency (ANSM) submits its report with the Health Insurance. The document should shed new light on the situation.
On this occasion, the Director General of Health, Benoît Vallet, meets the associations of victims. Between complaints and mea culpa, families call to establish responsibilities. Among the accused: the health authorities who have belatedly recognized the teratogenic effects of sodium valproate; the laboratory that reported the risks to the fetus late; prescribers who were able to establish the prescriptions without always informing the patients. Back on a thorny issue in 5 key dates.
May 28, 2015: the first complaint. In the middle of 2015, a family stepped up to the plate. She files a complaint against the Sanofi laboratory, which produces Dépakine. In total, around thirty homes are defended by Me Charles Joseph-Oudin. He denounces the continuation of prescriptions until 2010 despite its effects known from 1980. The revelations to come will be much more worrying.
October 13, 2015: the investigation of the Paris prosecutor’s office. After the complaints, the investigation. The Paris prosecutor’s office is launching a preliminary investigation. It aims to establish the responsibilities between the various laboratories which produce Depakine and its generics (Sanofi, Zentiva, Biogaran, Aguettant, Teva, Sandoz, Arrow, Mylan, EG Labo) and the health authorities. The prescribers are then not questioned.
February 23, 2016: the delay of the authorities. The General Inspectorate of Social Affairs (Igas) broadens the spectrum of officials with a corrosive report. It reports 450 child victims of Dépakine in the Rhône-Alpes region between 2006 and 2014. 93,000 patients received the anticonvulsant. The rapporteurs point to the delay of the health authorities in admitting the teratogenic effects of the drug and in communicating on this subject. The “lack of responsiveness” is obvious: before 2010, only an invitation to consult the doctor in the event of pregnancy is written on the leaflet. But the Igas does not forget the prescribers, who inform patients poorly or little.
March 7, 2016: possible compensation for victims. The report from the Igas visibly pushed the Ministry of Health to step up the pace. Marisol Touraine meets the families and makes several announcements. In the first place: the possible compensation of families after a mission of legal expertise. A new pictogram, clearer and more visible, will also be displayed. In order to support prescribers, information will be posted on prescription assistance devices and information letters will be distributed.
August 11, 2016: 10 00 women would be affected. In the columns of Chained Duck, a first estimate of the Dépakine scandal is delivered. According to the report (submitted on August 24) 10,000 pregnant women received Depakine between 2007 and 2014. The study should be followed by an action plan submitted in early September.

.