In 2014, more than 1,300 women received Depakine during their pregnancy. The teratogenic effects of this anticonvulsant have however been known for several decades.

- More than 1,300 women received Depakine during their pregnancy in 2014.
- Sodium valproate, its active substance, is indicated for epilepsy and bipolar disorder as a second-line treatment.
- The teratogenic effects of this drug have been identified since 1980. Its impact on neuro-behavioral disorders was raised in the 2000s.
- It was not until 2010 that the patient leaflets were amended to reflect the risks.
- The statute of limitations is now restricted and an agreement form must be signed.
The decrease in Depakine prescriptions did take place, but not enough. the eagerly awaited report of the National Medicines Safety Agency (ANSM) was handed over to the Ministry of Health on August 24. It calls into question the effectiveness of the risk reduction strategy deployed since 2010 for women of childbearing age. Despite the danger to the fetus, 14,322 women received this treatment between 2007 and 2014, the document reveals. (8,204 for treating epilepsy, 6,149 for bipolar disorder).
Teratogenic and at the origin of neurobehavioural disorders – including autism – the prescription of Depakine must be carefully monitored by pregnant women. But the French recommendations have not been heard enough from doctors. In 2014, 1,333 epilepsy or bipolar patients began their pregnancy while still taking sodium valproate. At the origin of these prescriptions are mainly liberal general practitioners and hospital professionals.
85% exposure to 1e trimester
This figure shows a significant decline: between 2007 and 2014, the number of so-called “exposed” gestations decreased by 42%. Women of childbearing age are also less likely to benefit from this treatment. The alternative use of other molecules has indeed seen a marked increase over the same period.
But this remains largely insufficient, in view of the data from the Health Insurance. “These results suggest that the application of risk reduction measures needs to be strengthened,” the report stresses. It is essential to extend the monitoring of exposure to other treatments for epilepsy and bipolar disorders, ”he adds.
The details of the study are more worrying: 85% of children born to mothers with epilepsy were exposed to 1e and 2e quarters. It is during this time that teratogenic effects are most likely to occur. This share does not drop slightly between the beginning and the end of gestation: in two out of three cases, the prescription is maintained.
The picture is radically different for women with bipolar disorder. Almost all of them are still under Dépakote at 1e trimester compared to only 14% at the end of pregnancy.
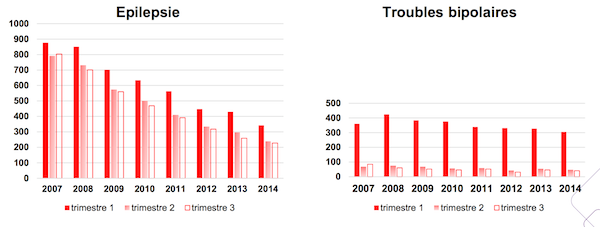
Source: ANSM study / Health insurance
8% miscarriages
Of the 14,322 pregnancies under Depakine, a majority (61%) resulted in a live birth. 30% of the patients chose to terminate their gestation. Only 115 fetuses are stillborn. In contrast, 8% of women experienced a spontaneous miscarriage or ectopic pregnancy, two known side effects of sodium valproate. The study does not specify whether this choice was motivated by knowledge of the teratogenic risks of the drug.
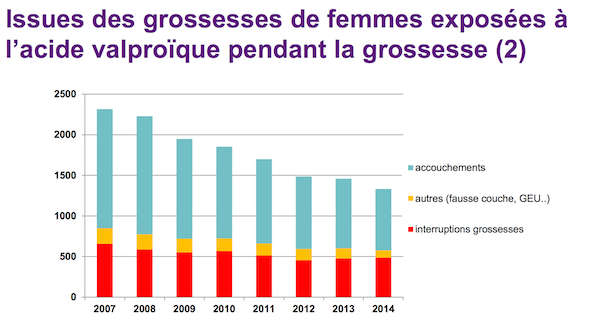
Source: ANSM study / Health insurance
In addition to the gap in the care of epilepsy and bipolar patients, this work highlights the potential health scandal surrounding Depakine. Its magnitude is not yet determined. The thousands of births exposed to this anticonvulsant are the subject of a second study by the ANSM. It aims to determine the impact of this prenatal exposure. The results will be submitted at the end of 2016. Without waiting for them, the Minister of Health Marisol Touraine announced a series of measures intended to further reduce pregnancies exposed to sodium valproate. The victims will also be compensated thanks to a law presented to Parliament at the end of the year.
.