Dental implants made from a raw material intended for industrial, not medical, use have been withdrawn from the market.
Implants, to say the least, suspect. The ANSM (National Medicines Safety Agency) has suspended the marketing and manufacture of medical devices designed by the company Ethical Medical Implants (EMI), based in Mérignac (Gironde). The materials concerned are implants, fillers, implant constructions and membranes, all intended for dental implantology.
In his health policing decision (DSP), the agency indicates that it carried out an inspection in November 2016 at the premises near Bordeaux. The inspectors then discovered that the raw material from which the implants were made should never have been used for medical purposes.
“Not for human implantation”
“The packaging of the reference raw material VESTAKEEP 4000P found in stock on the site of the company EMI bore a yellow labeling with a mention translating the prohibition to use this raw material as it is in human implantology (” this material is not for human implantation ”)”, notes the ANSM.
Implants are made from polyetheretherketone (PEEK), a material that is used for medical purposes after being repackaged for that purpose. Except that as it stands, the reference VESTAKEEP 4000P “appears on the list of raw materials intended for industrial use”, and not for medicine – and even less in implantology.
Biocompatibility and toxicity
As it stands, nothing can guarantee the biocompatibility of this medical device, which is not, nor its toxicity. This is despite the fact that such devices must be manufactured under strict procedures “for the reciprocal compatibility between the materials used and the tissues, cells and body fluids”, and in order to “minimize the risk posed by contaminants and residues”. The clinical and preclinical data provided by the company did not make it possible to “demonstrate the safety and health performance” of the equipment.
In addition, the ANSM notes failures in the process of sterilization and storage of devices. The agency asks the manufacturer to withdraw the targeted products from the market and to contact professionals and companies likely to hold these products.
According to the site societe.com, the Ethical Medical Implants company is a very small structure, with three to five employees, whose turnover in 2015 was 7,000 euros. Not enough to irrigate the French market, therefore. Contacted, the agency indicates that regarding the risk, it has been assessed for patients already implanted. Elements transmitted to the ANSM made it possible to demonstrate the absence of risk of toxicity of the raw material used. In addition, the ANSM has no material vigilance report concerning this medical device.
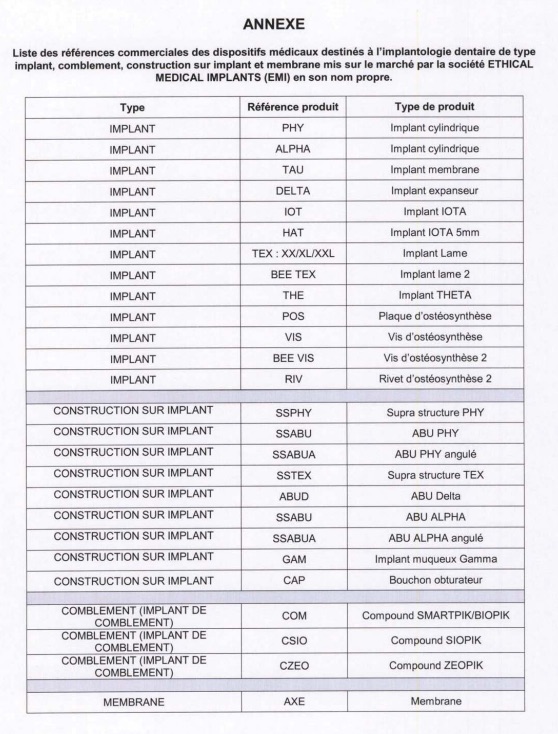
.