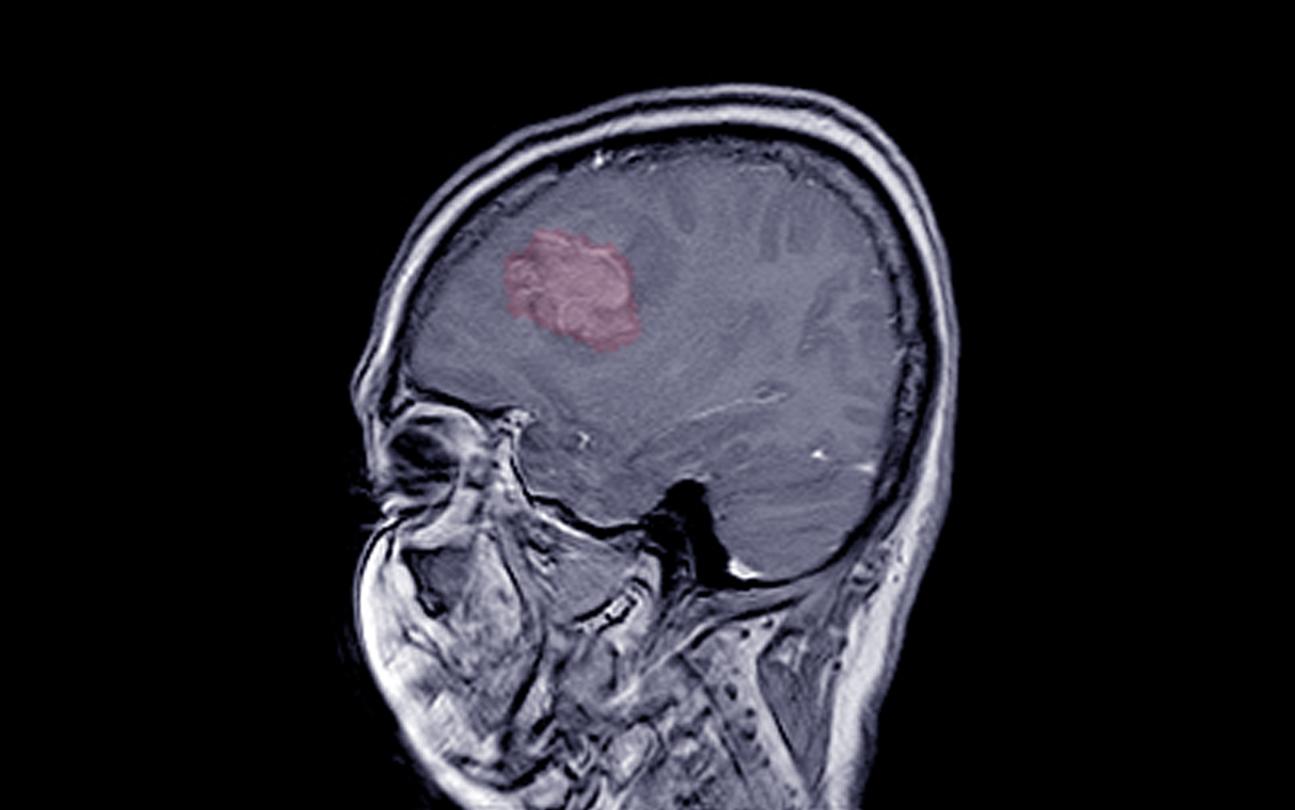
Prescribed since the 1980s as a contraceptive or treatment against acne, Androcur is today suspected of greatly increasing the risk of brain tumours. From July 1, this information must be sent to any new patient starting treatment.
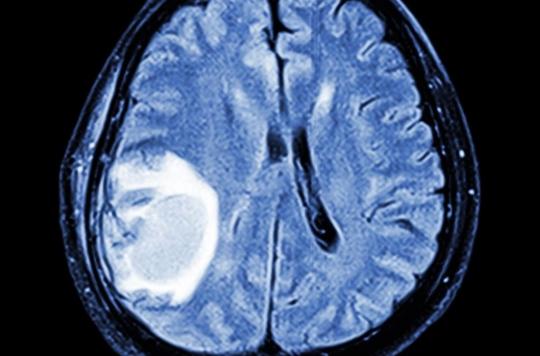
Marketed since the 1980s by Bayer laboratories, Androcur is a progestogen prescribed for years to women suffering from hirsutism, extreme hair growth, against acne, endometriosis or as a form of contraception. Belonging to the class of steroidal antiandrogens and containing an active ingredient called cyproterone acetate, Androcur is also used in men to treat advanced prostate cancer.
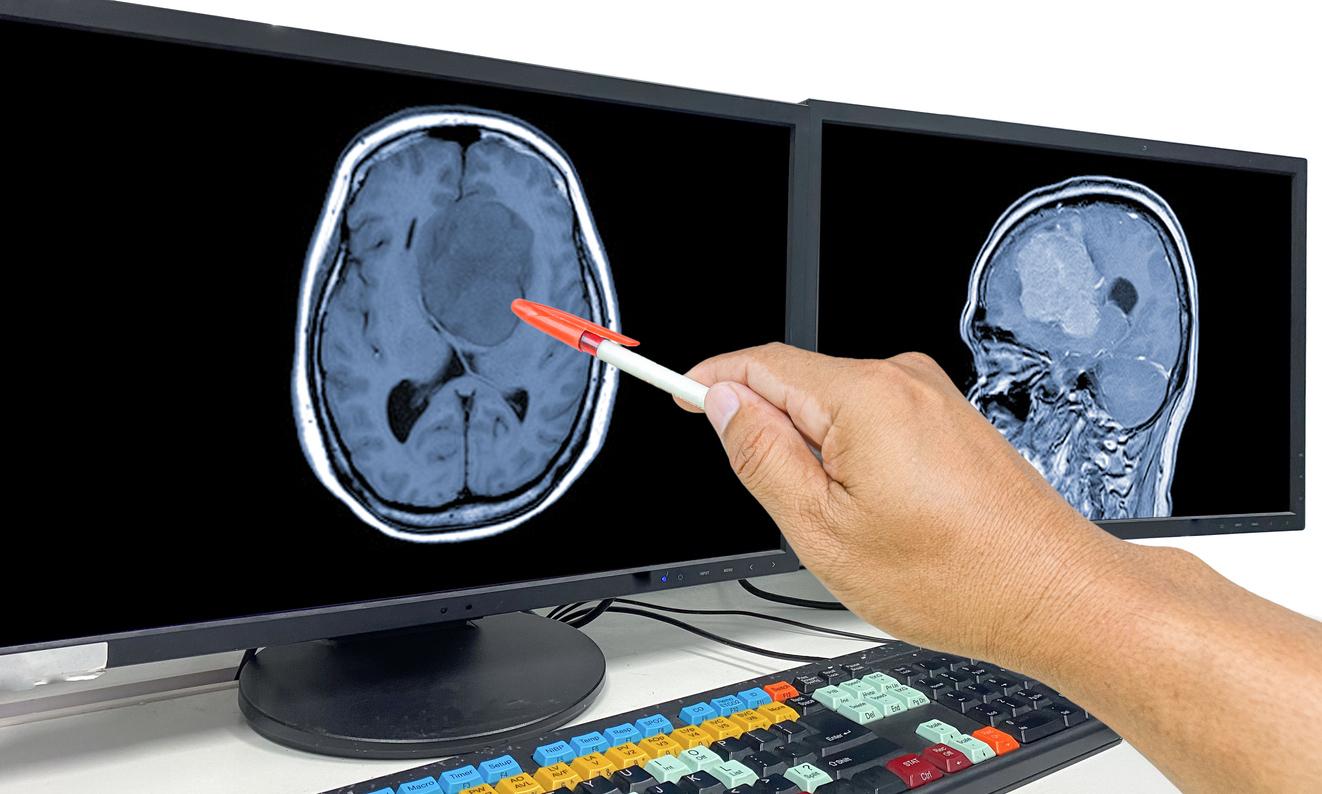
But in August 2018, a study conducted by Health Insurance reveals that this drug is suspected of multiplying up to 20 (after 5 years of treatment), and even more, the probability of meningiomas, that is to say intracranial tumors, in women treated for a long time and at high doses. Generally benign, these tumors can cause serious sequelae such as memory problems, epilepsy or loss of taste and smell.
An obligation to inform and monitor patients
From 1er July, any new patient starting treatment with Androcur or one of its generics must be systematically informed of the risk of tumor linked to its prolonged use, informed the National Medicines Agency (ANSM). From now on, an information sheet on the risk of meningioma will therefore have to be given to patients. The latter will only be able to obtain Androcur and its generics in pharmacies on the mandatory presentation of an annual information certificate that they will have signed as well as the prescribing doctor, from July 1 for new treatments and from January 1 2020 for renewals. The validity of the prescription must be reassessed each year.
“This information mainly concerns women, cyproterone acetate, the active ingredient of these drugs, being prescribed to men in the minority, in particular for prostate cancer and sexual deviance (chemical castration for pedophilia and / or repeated rape), “explains to AFP Dr. Jean-Michel Race, endocrinologist, ANSM.
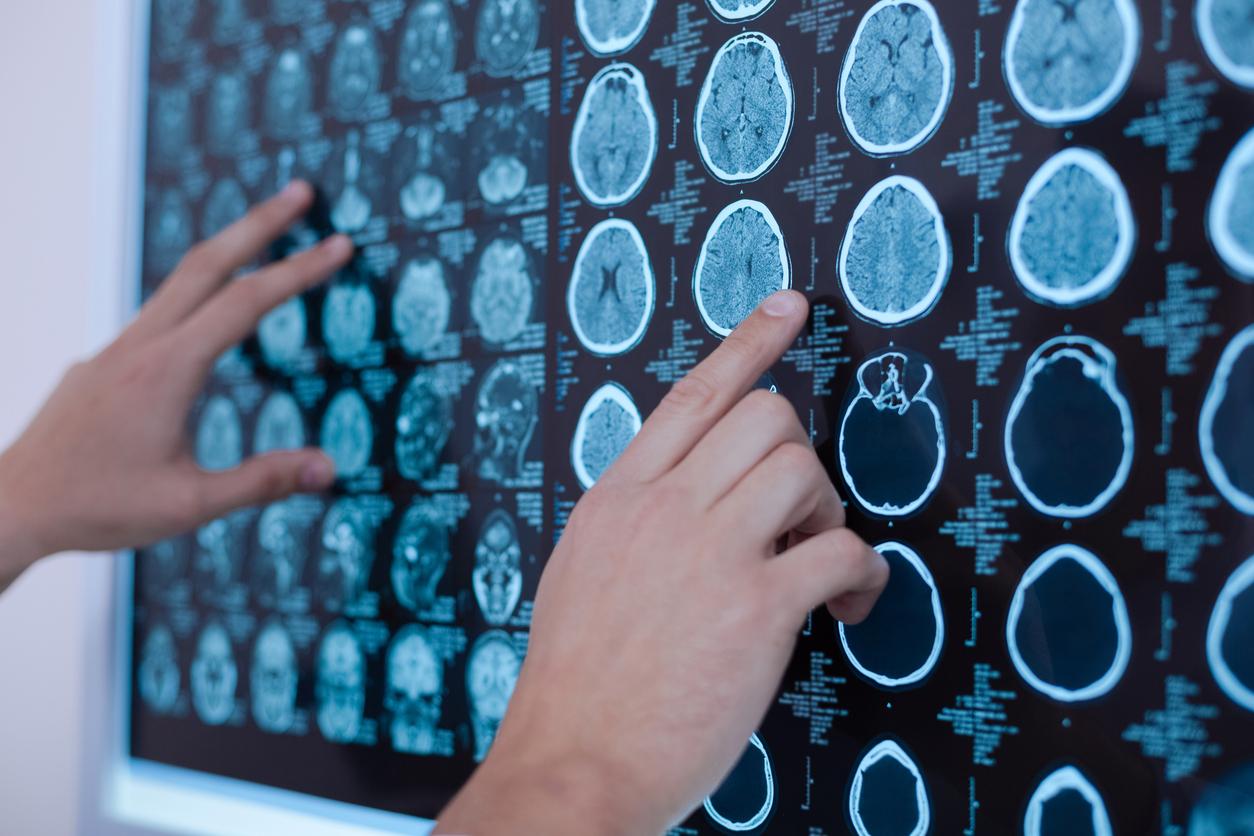
A cerebral MRI should systematically be prescribed for patients starting their treatment, and considered for the continuation of treatment. As long as the treatment is maintained, the next MRI will take place no later than 5 years after the first, then every two years if the previous MRI is normal.
At least 500 meningiomas attributable to Androcur
For its part, the Health Insurance is continuing its investigations to better understand the link between Androcur and the appearance of brain tumours. A pharmacovigilance survey has been commissioned and its results will be discussed on June 18 at the meeting of the Pharmacovigilance Technical Committee.
Last March, the Cnam had published a summary in which it estimated at least 500 the number of meningiomas operated on or treated by radiotherapy attributable to prolonged exposure to Androcur at high doses.
Pending further information, and to answer questions from patients and their families, the ANSM has made available a toll-free number (0 805 04 01 10) accessible free of charge from Monday to Friday from 9 a.m. to 7 p.m.
.