The National Medicines Safety Agency (ANSM) has decided to measure the health benefit of therapeutic cannabis in France. If allowed, it could alleviate the suffering of around 300,000 people. Some have already taken the liberty of cultivating it to soothe their pain.

The legalization of therapeutic cannabis in France is still a subject of debate. After announcing that medical cannabis “could arrive in France”, the Minister of Health Agnès Buzyn had taken a step closer to “the cannabis cigarette” for medical use during an interview with RMC on July 10, on the condition, however, that it brings “a plus” in the treatment of diseases compared to cannabis-based drugs already authorized in France.
Today, the National Medicines Safety Agency (ANSM) decided to launch an evaluation to measure the interest of prescribing therapeutic cannabis. “This is a real demand from patients, it is also a real demand from doctors who wish to develop this therapeutic use of cannabis,” Nathalie Richard, of the Medicines Agency, told France Info.
A committee was created to “assess the relevance of developing the therapeutic use of cannabis in France” and will deliver its opinion in December. “The indication systematically adopted is the use in the case of neurological pain and those related to cancer”, explains Nathalie Richard. “It will be the role of the committee to define more precise indications”.
300,000 French people could need it
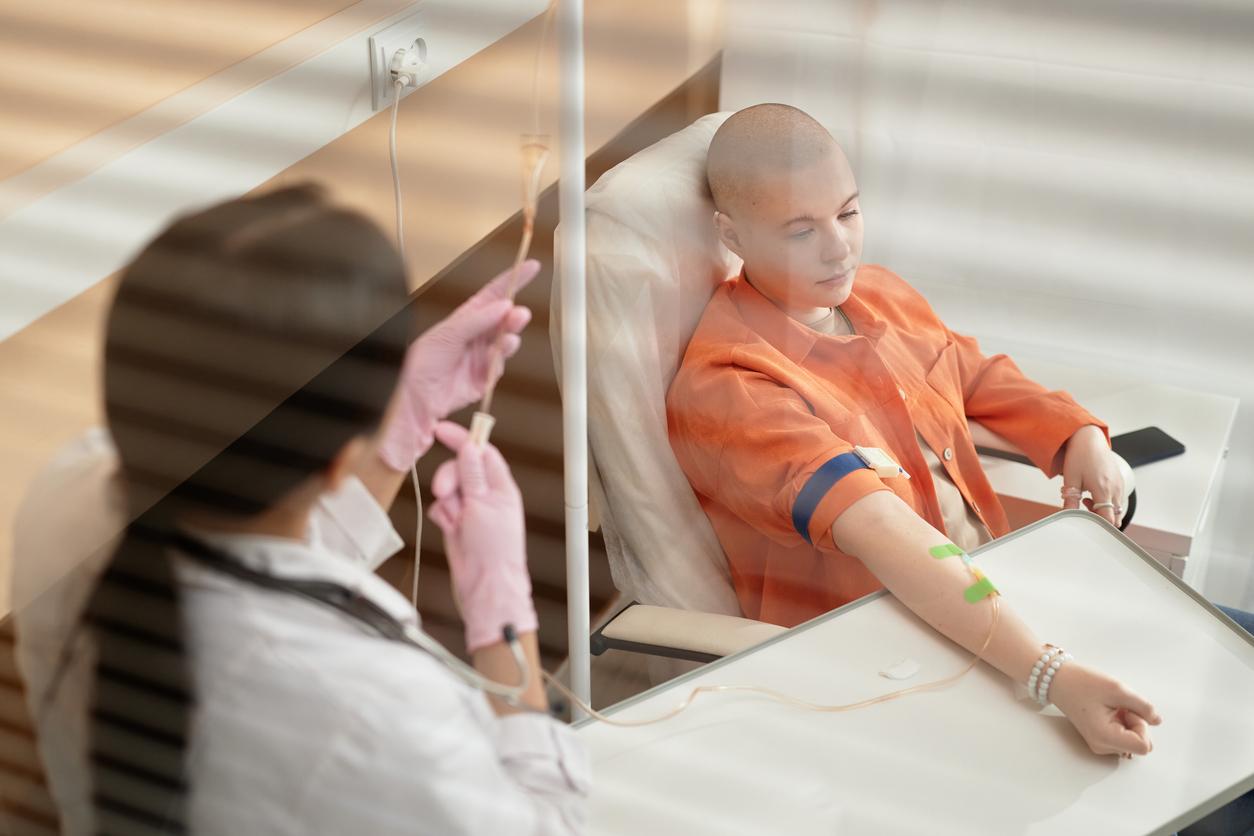
It is estimated that 300,000 people could need to resort to therapeutic cannabis to alleviate their suffering if it were ever authorized in France. “For me, it was miraculous”, explains to Europe 1 Corinne, suffering from ankylosing spondylitis, a kind of very painful rheumatism. “If I am in a crisis and I cannot move at all, I vaporize – because I do not smoke – and there it takes effect very quickly. Then I feel the pains in a distant way and I can bear them”.
At 50, Corinne cultivates and harvests her own cannabis. “The varieties that you find on the black market are varieties that are made to get high, to have your head elsewhere and me is not at all what suits me. What I need is a very strong anti-inflammatory effect “.
In cannabis, there are 2 main molecules of interest among a hundred: THC, or tetrahydrocannabidiol, the psychoactive substance whose planing effects are sought, and cannabidiol, whose sedative and relaxing properties have been validated (in addition to the usual treatment) in nausea in cancer patients and in certain rare forms of epilepsy.
American oncologists and medical cannabis
In the United States, where cannabis is permitted in some states and cannabidiol in 28, 80% of oncologists say they have already discussed the issue of medical cannabis with their patients, but less than 30% of them believe they have enough scientific evidence to make such recommendations. “The scientific evidence supporting the use of medical marijuana in oncology is still very thin, which puts physicians in a very uncomfortable position,” said Dr Ilana Braun of the Dana-Farber Institute of Adult Psychosocial Oncology.
Indeed, so far, no randomized clinical trial has looked at the effects of medical marijuana in cancer patients, other than its effects on nausea, so oncologists are relying on that. research into the use of cannabis for medical purposes in the treatment of diseases other than cancer.
Yet two-thirds of oncologists surveyed believe that medical marijuana is an effective adjunct to standard pain treatment. In their experience, therapeutic cannabis is just as, if not more effective than conventional treatments against the side effects of chemotherapy such as nausea or lack of appetite.
.