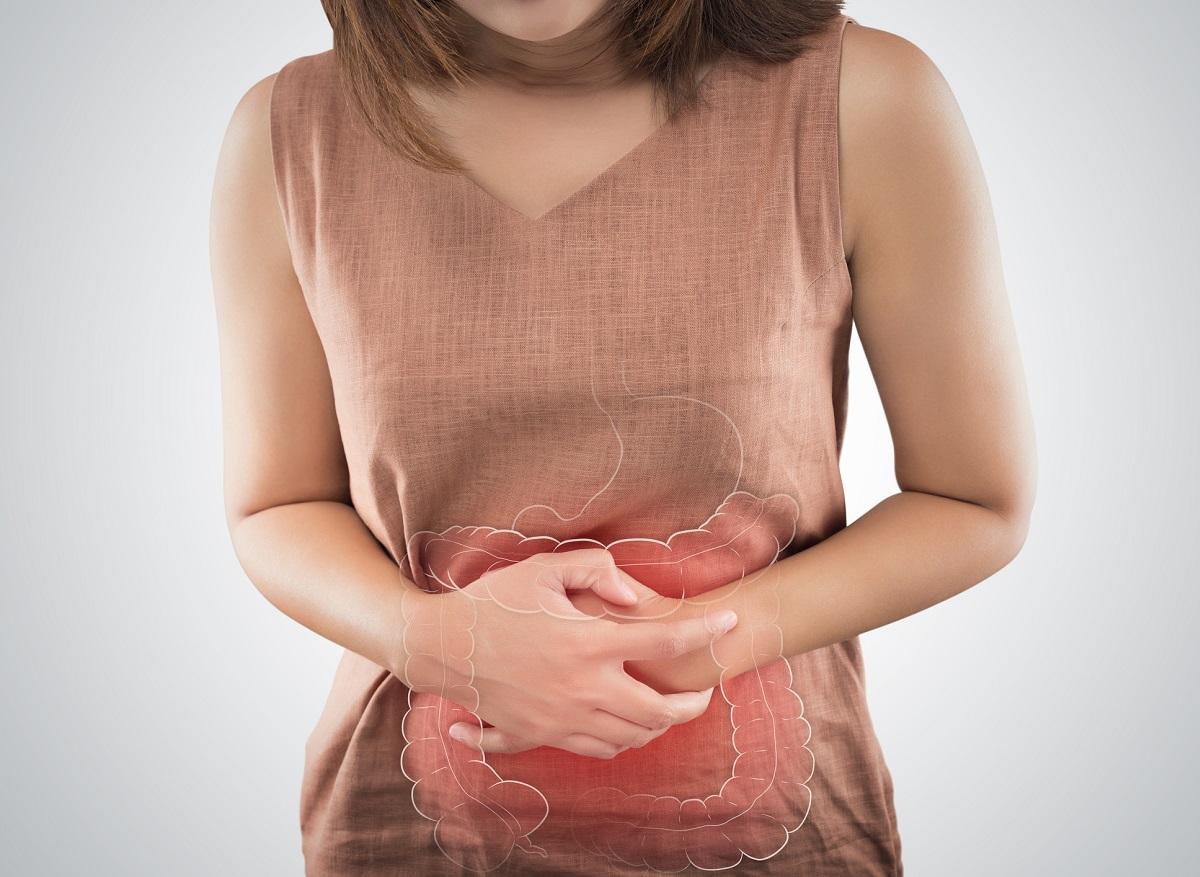
Several users of Ozempic have filed complaints against its manufacturer due to complications caused by the anti-diabetes drug, also used for weight loss: one of the plaintiffs will suffer from diarrhea for the rest of her life.
- The Daily Mail reveals that there are more than forty lawsuits against the manufacturer of Ozempic in the USA due to problems reported after taking the drug. The files will be brought together in January to be judged together.
- One of the complainants explains that she will suffer from chronic diarrhea after developing an “intestinal injury”.
- Brea Hand, 23, had to be treated in intensive care after developing gastroparesis and diabetic ketoacidosis.
A investigation of DailyMail.com reveals that Novo Nordisk, the manufacturer of theOzempic and of Wegovyis being sued by dozens of American patients. They assure that after taking the anti-diabetic, known to facilitate weight loss, they suffered significant side effects of which they were not warned.
Ozempic : several complaints due to complications
The British daily examined more than a dozen lawsuits filed against Novo Nordisk since November. Most complainants report having developed gastroparesis. This digestive disease affects the movements of the stomach muscles and causes a delay in gastric emptying of solids. It causes nausea, bloating, severe abdominal pain or even burnsstomach. These symptoms can cause weight loss and nutritional deficiencies.
Among the ongoing procedures, a woman whose identity has not been revealed, indicates having been diagnosed with a “life-threatening intestinal injury” after using Ozempic. To save his badly affected colon, surgeons had to carry out an 8-hour operation. If she survived the surgery, doctors warned she would suffer “for the rest of his life” and that she “will never have solid stools again” (chronic diarrhea). The patient accuses Novo Nordisk of “failing to properly warn of the risk of gastroparesis on the packaging of medicines”.
Currently, American justice has more than 40 cases against Novo Nordisk. Some complaints also target Eli Lilly, the manufacturer of Mounjaroa diabetes medication that works in the same way asOzempic and the Wegovy. Additionally, lawyers are reviewing thousands of other potential cases. THE Daily Email reveals that all complaints should be grouped this month into multidistrict litigation (a procedure in the USA which allows several similar cases to be centralized before a single judge).
Lawyer Cameron Stephenson, whose firm has handled several plaintiffs, explained to the British newspaper: “There is no doubt in my mind that there will be thousands of cases that will be filed in the “multidistrict litigation” over time.”

Ozempic : “I have never experienced this kind of pain in all my life”
Brea Hand, a 23-year-old plaintiff who lives in Oklahoma, its entrusted At DailyMail.com. She started usingOzempic in May 2023 to control his fluctuating weight and prediabetes. After a few weeks, she experienced nausea, vomiting and constipation. After five hospital visits, doctors diagnosed him with gastroparesis and diabetic ketoacidosis. His state of health required intensive care during his last visit.
“The doctors said my body had such a high rate of acidosis that if I had waited another day I would not have survived.”explains the mother of two children whose lawsuit was filed on December 28. “It was scary. It was painful. I have never experienced this kind of pain in my entire life and I never want to experience this again.”she confides.
The young woman claims not to have been informed of the side effects and risks of theOzempic. “I wouldn’t recommend it to anyone, personally. Just taking that risk would be too much for me based on what I’ve experienced.”she adds.
In a statement to DailyMail.comNovo Nordisk indicates: “Novo Nordisk believes that the allegations in the lawsuit are without merit, and we intend to vigorously defend ourselves against these allegations. Patient safety is our top priority at Novo Nordisk, and we work closely with the Food and drug Administration (US Food and Drug Administration) to continuously monitor the safety profile of our medicines.”