The French Association of Thyroid Patients (AFMT) claims to have ordered an analysis of the new formula of Levothyrox from a foreign laboratory. The results show the underdosing of one molecule and the presence of another, not present in the list of components. This would explain, according to the association, the side effects from which many patients suffer.
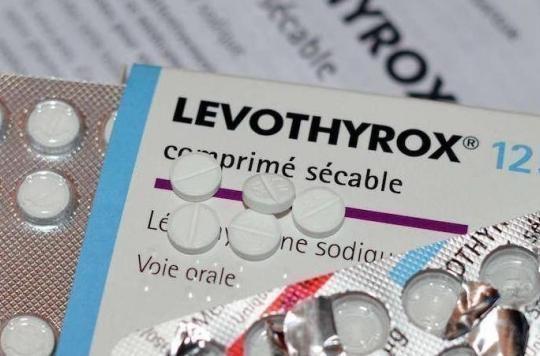
The battle of thyroid patients against the new formula of Levothyrox continues. While she had already ordered in early May an analysis showing metal nanoparticles in the new drug formula from the Merck laboratory, French Association of Thyroid Patients (AFMT) continues its fight.
In a statement dated June 14 and relayed by FranceInfo, the association says it has ordered a new analysis of the controversial drug formula from a foreign laboratory. According to her, the results highlight two notable changes from the previous formula.
Awakening from sleeping cancers and side effects
First, it would contain less levothyroxine than the specifications in force. According to the AFMT, the slightest presence of this synthetic thyroid hormone would be at the origin of the malfunctions of the treatment observed in the patients. “Cancer patients are underdosed in thyroid hormones, we have consistently observed awakenings from dormant cancers for years,” says the association.
In addition, according to the association, the analysis ordered by the association shows the presence in the drug of dextrothyroxine while it is not in the list of components. Not marketed in France, this synthetic substance has been banned in the United States because it is suspected of causing the same side effects as those currently complained of by thyroid patients using the new formula of Levothyrox.
The AMFT calls for the resignation of Agnès Buzyn
The AMFT has indicated that the results of the study of the new formula of Levothyrox have been transmitted to an investigating judge of the Marseille health center in charge of the case, opened for “aggravated deception, involuntary injuries and endangerment of ‘others’.
Asked by FranceInfo, Chantal L’Hoir, president of the French Association of Thyroid Patients, calls for the resignation of the Minister of Health Agnès Buzyn, as well as the “urgent” recognition of a health crisis “and, as a precautionary principle , upon withdrawal of Levothyrox “.
“There were three and a half million lots sold at the time, per month. (…) So we cannot take any risks. In a country which calls itself scientific, it is not worthy,” he believes -she. “The Ministry of Health has only to recognize a health crisis with an emergency, for the reimbursement for example of the TCAPS [alternative au Levothyrox du laboratoire Génévrier] that they blocked. “
A statement “scientifically unfounded” according to Merck
For its part, the Merck laboratory, which markets the new formula of Levothyrox, said in a press release that the AMFT’s statement was “scientifically unfounded”.
“We formally deny the presence of a dextrorotatory form in the tablets of Levothyrox, whether it is the old or the new formula (…) Concerning the presence of a different form of levothyroxine in the new formula of Levothyrox (Dextrorotatory D-T4 form), we formally affirm that this is not the case.We recall that the active substance used for Levothyrox new formula is strictly identical to that present in the old formula of Levothyrox (Levothyrox form known as L -T4 molecular form) “, continues the laboratory through the voice of Valérie Leto, Pharmacist in charge of Merck and author of the press release.
The Levotyrox case began in February 2017, when the formulation of the drug was changed. This change consisted of replacing the lactose, which coated the thyroid hormone, levothyroxine, into a tablet, with mannitol. This modification had been requested by the Medicines Agency (ANSM) in order to guarantee the stability of the product over time, which was not the case with the old formula.
But the patients experienced unwanted side effects. Of the 2.3 million patients treated in France, 17,000 cases of side effects have been identified. In total, 5,062 adverse effects were classified as serious and 14 deaths were recorded by the ANSM, without a direct link with the new formula being able to be formally established. According to the Ministry of Health, 500,000 people have abandoned the new formula of the drug.

.