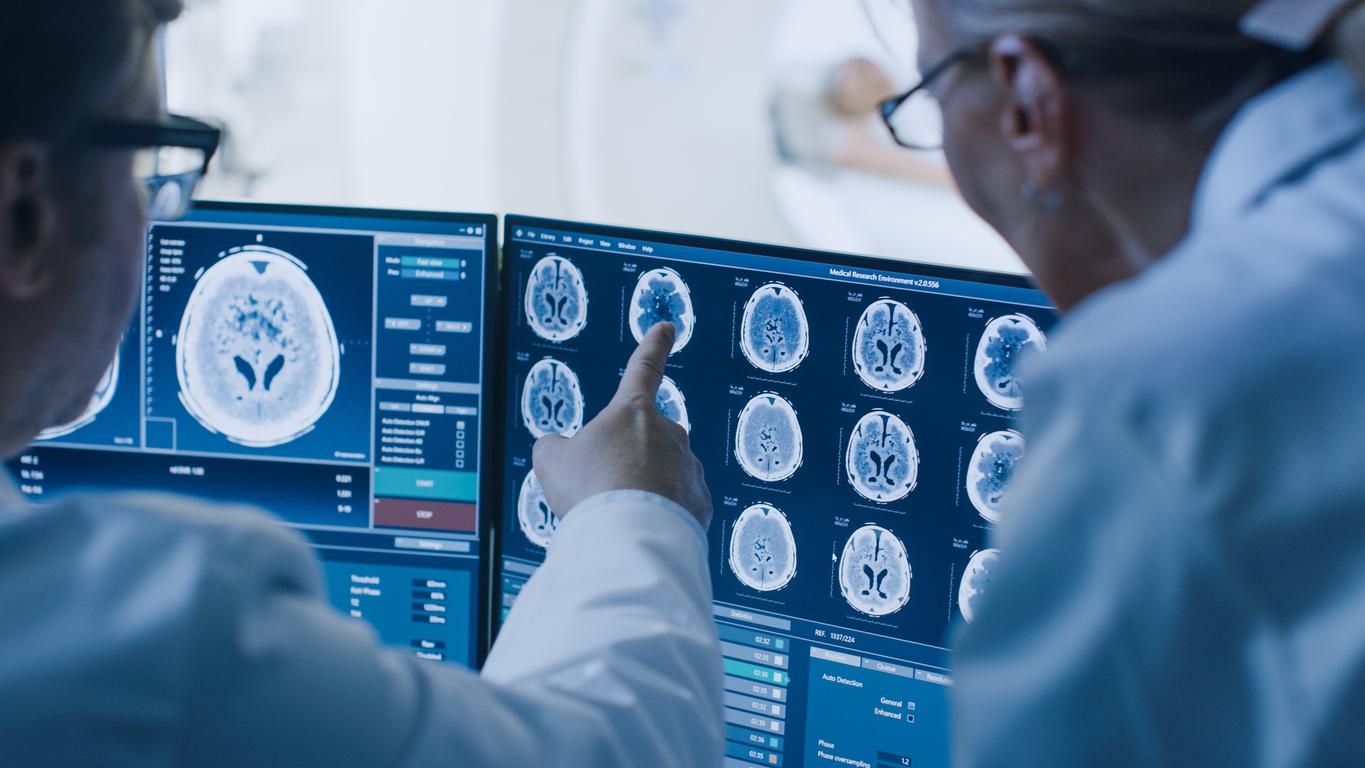
American researchers have succeeded in “correcting” the DNA of human embryos to remove a mutation responsible for an inherited disease.

Between hopes and fears. This is where CRISPR-cas 9 is located, the gene editing technique, developed by Emmanuelle Charpentier and Jennifer Doudna. The method is “simple” (compared to other genetic engineering techniques!) And inexpensive: two strong arguments to seduce researchers. But above all, it is very powerful and could, in absolute terms, make it possible to “correct” genetic anomalies with serious, even lethal consequences.
So would these molecular scissors sign the end of genetic diseases? Maybe. But there is often a long way to go from experimental results to clinical applications. However, there, everything goes quickly, very quickly, too fast for some. After research carried out in China which had moved the international scientific community, this time it was researchers from Portland who announced that they had succeeded in correcting a mutation responsible for serious heart disease in human embryos.
Confirmed effectiveness
Those works, published in the scientific journal Nature, focused on an inherited form of hypertrophic cardiomyopathy. This disease, which leads to the thickening of the walls of the heart, can cause severe symptoms and even sudden death.
Shoukhrat Mitalipov and his colleagues at Oregon Health & Science University fertilized oocytes from healthy donors with sperm from a man with a mutation in the MYBPC3 gene, known to be responsible for the disease. The scientists then compared the fate of these embryos after 3 days of development, with or without CRISPR-cas9 treatment.
In total, a little more than 50 “edited” embryos were compared to around 20 control embryos. And the scientists observed that the technique was very effective: around two-thirds of the embryos treated contained two normal copies of the MYBPC3 gene, compared with only 50% for the control embryos.
Crucial timing
The researchers took their investigations further and succeeded in showing that the moment at which the embryos were processed was of great importance. Using CRISPR-cas9 as soon as the ova and spermatozoa come into contact would make it possible to avoid the production of “mosaics”, ie embryos containing both healthy cells and mutated cells. However, these mosaics constitute one of the major obstacles to the use of CRISPR-cas9 in humans. Another limitation of the method: the induction of modifications of the “off-target” genome. But there again, the American researchers specify that a complete sequencing of the genome did not show any undesirable mutation among the treated embryos.
These results undoubtedly constitute a major advance in the knowledge and mastery of CRISPR-cas9. One of the co-authors of the study, Sanjiv Kaul, believes that the method could be transposed to many mutations, in particular those affecting the BRCA1 and BRCA2 genes, reports Paul Benkimoun in The world. But scientists keep a cool head: “Gene editing must still be optimized before clinical application to germ cells can be considered,” they say in their publication.
One of the limitations to the development of CRISPR could come from funding. If the United States allows research on the human embryo, it cannot receive public funding. In France, the National Academy of Medicine adopted in 2016 a position favorable to the development of research using CRISPR, even on the human embryo. But French law still prohibits “any intervention on the structure of DNA having the consequence of modifying the genome of the offspring”. The next breakthrough may come from the UK, which has passed more permissive legislation on the issue.
.