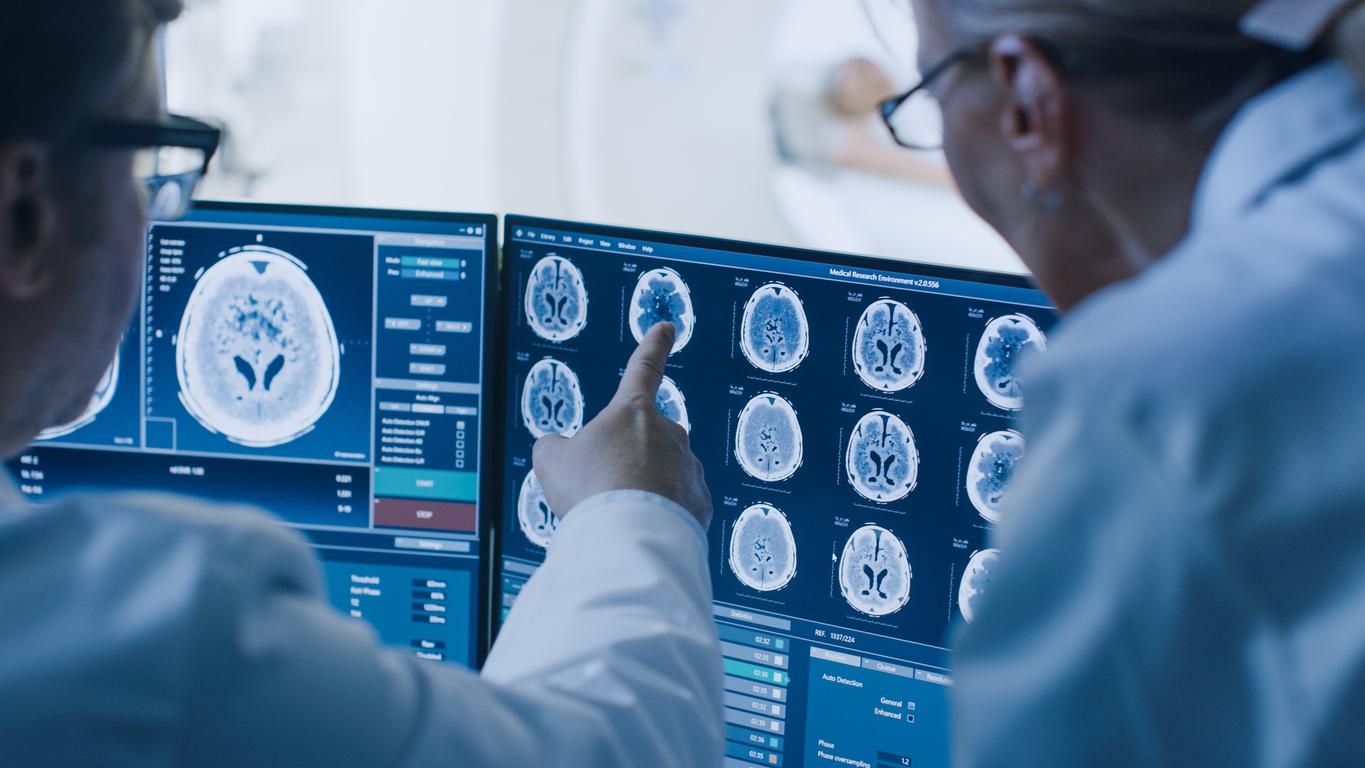
November 06, 2015. Americans will soon be able to find out if they carry genetic mutations that would increase the risk of disease in their offspring. DNA tests will soon be available over the counter.
A first abortive attempt in 2013
In 2013, 23andMe struck hard by giving everyone the opportunity to know their personal genetic information, without going through the doctor’s box. Shortly after, the US Food and Drug Administration (FDA) shut down sales of the start-up. The analysis of the risks of cancer, cardiovascular diseases, Alzheimer’s and diabetes posed problems on potential consequences on health. False positive or false negative results could then lead to inappropriate treatment or, on the contrary, to ignorance of a real risk.
The return of the DNA analysis kit
Two later, 23andMe was given the green light to market a new test, which no longer allows to assess the risks of which the FDA was concerned. “We have worked with the FDA for almost two years to establish a regulatory framework that allows direct sale to consumers of genetic tests,” said Anne Wojcicki, CEO of the company. These new kits will allow their customers to quickly find out whether they are carriers of genetic mutations that can greatly increase the risk that their children will have certain diseases, such as cystic fibrosis.