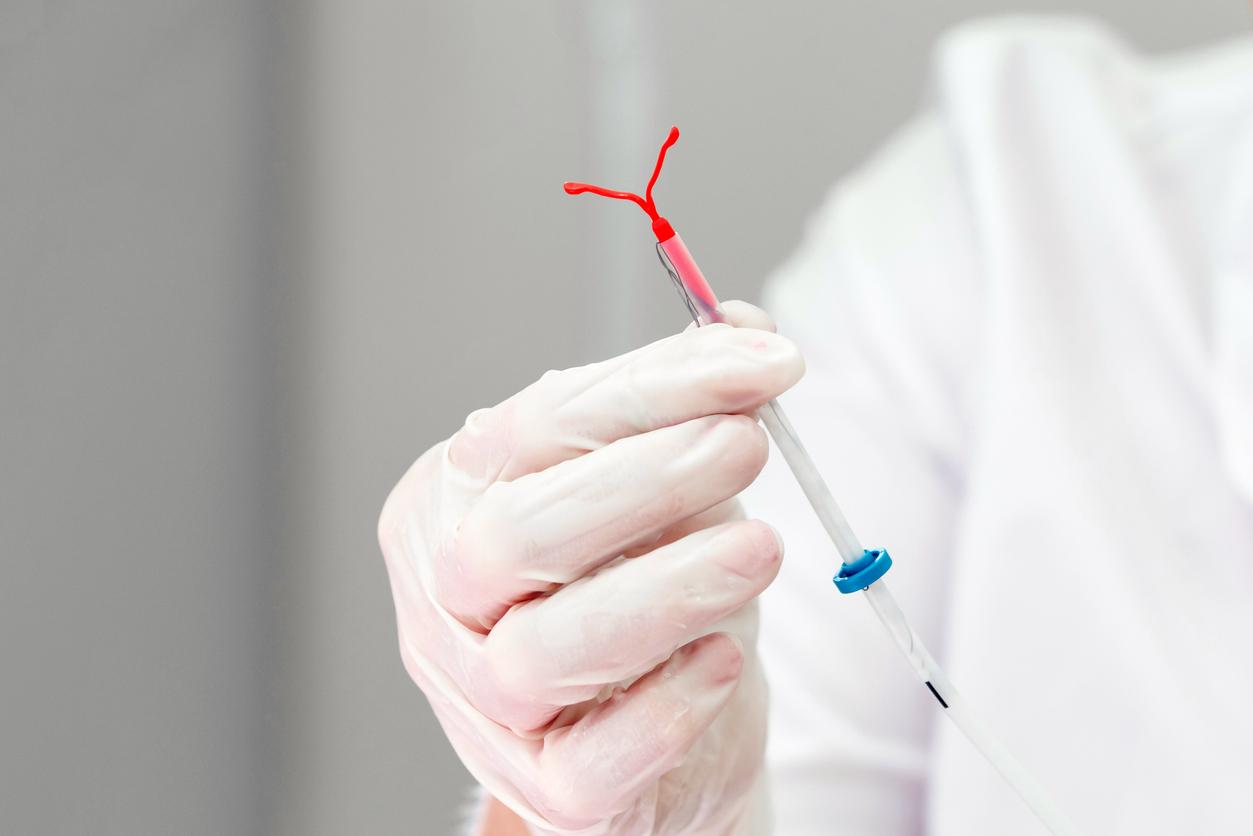
Definitive contraception Essure has been under national surveillance for two years. Two women decided to take legal action for side effects.

Implants under close surveillance. The Essure method of definitive contraception will end up in court. Charles Joseph-Oudin defends two patients who were victims of severe complications. They are attributed to this medical device which obstructs the fallopian tubes. According to The Parisian, which reveals the case, the women suffered from symptoms ranging as far as depression. 120,000 women have chosen the Essure method since 2002, when it was put on the market. The manufacturer of the implants, Bayer, is implicated. The Parisian lawyer asks that the victims be compensated.
An increase in reports
The contraceptive implant has in fact been under close surveillance since 2014. Several health agencies have deployed measures with patients and obstetrician-gynecologists. In fact, medical device vigilance reports are on the rise. They went from 42 for the year 2012 and 162 for the first three quarters of 2016.
The National Medicines Safety Agency (ANSM), contacted by Why actor, specifies that these figures do not reflect the number of incidents that have occurred each year. “In 2016, more than a third of the declarations are retroactive”, underlines the Agency. The lifts mainly concern difficulties that arose during installation. But abdominal or pelvic pain, bleeding and pregnancy can also occur.
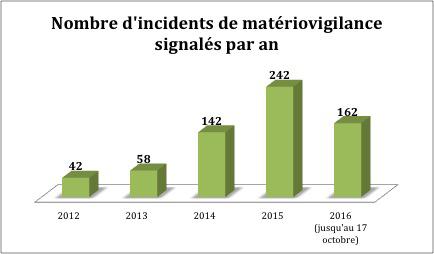
But the increase is real, and requires investigation. What the ANSM did in July 2015. Several investigations concerning adverse effects have been launched. They show that it is the way in which the installation is carried out that poses the problem and not the device. Another study compares the safety of the Essure method versus conventional tubal ligation. The results are expected for the first quarter of 2017.
Fewer empowered professionals
The health authorities did not content themselves with evaluating the extent of the phenomenon. They also asked health professionals to change their practices. As of November 2015, the Haute Autorité de Santé (HAS) has drawn up best practice recommendations, in conjunction with learned societies. A few months later, it was the ANSM’s turn to demand adjustments.
The Bayer laboratory must develop a leaflet for patients. The obstetrician-gynecologist must put it back on before each installation. The document details the risks associated with the device and the need for a follow-up consultation after three months. It’s done.
The Agency also wants to restrict the number of healthcare professionals authorized to use the Essure method. The Ministry of Health follows this advice: in February 2016, it publishes a decree reducing the establishments authorized to set up. A measure supposed to reduce reports of difficulties during installation. The documents, on the other hand, say nothing of the psychological complications pointed out by Charles Joseph-Oudin.
Definitive and irreversible contraception
The “Essure method” is a medical sterilization device. Available in France since 2002, it is final and above all irreversible. The implant developed by the Bayer laboratory is placed by an obstetrician-gynecologist in the fallopian tubes. This foreign body causes fibrosis there which ends up completely obstructing this reproductive organ, after about three months. A consultation makes it possible to monitor the effectiveness of this intervention. In the meantime, patients should continue to take another method of contraception.
.