Faced with the emergency, the health authorities of the Democratic Republic of Congo (DRC) and the World Health Organization (WHO) are opting for experimental treatments, the undesirable side effects of which are not known.

The re-emergence of the Ebola virus in the Democratic Republic of Congo (DRC) – the ninth since the 1970s – worries the authorities. If the first cases were declared in the district of Bikoro, 600 km from Kinshasa, the epidemic then spread in urban areas, in particular in the city of Mbandaka (1.2 million inhabitants) as well as in other villages of the province.
the June 3 review of the World Health Organization (WHO) reports 56 cases, including 25 deaths in Bikoro, Iboko and Wangata. “Of these 56 cases, 37 were laboratory confirmed, 13 are probable cases (deaths for which it was not possible to collect laboratory samples or do tests) and six are suspected cases “, indicates the ‘WHO which details the number of cases by locality: “Iboko (23 confirmed cases, 2 probable cases and 5 deaths), Bikoro (10 confirmed cases, 11 probable cases, 5 suspected cases and 17 deaths) and Wangata (4 confirmed cases, 1 suspected case and 3 deaths). As of May 31, 2018, 880 contacts (of infected people, Editor’s note) remain actively monitored “.
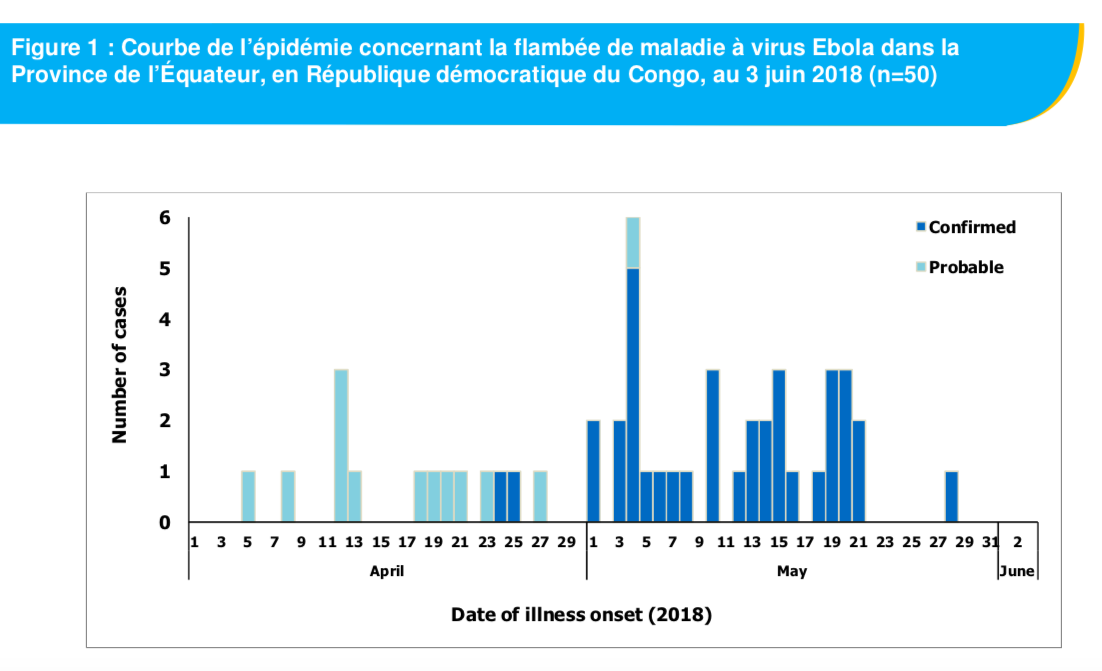
Credit: screenshot / situation report n ° 8 / WHO – June 3, 2018
Administration of an experimental vaccine
It is in the face of the risk of spread to densely populated cities such as the capital Kinshasa (11.5 million inhabitants) that the authorities have given the green light to the World Health Organization (WHO) to ship the first doses. of an experimental vaccine in mid-May. “If a city of this size is affected by Ebola disease, there will be a significant urban focus, which will be a real challenge,” World Peter Salama, who heads emergency operations at WHO: “When Ebola spreads to urban areas, especially slums, it is extremely difficult to overcome the disease.” And whether the epidemic takes on the appearance of a global pandemic depends only on the responsiveness of the country’s health authorities. The Democratic Republic of the Congo has therefore started a race against time to get ahead of the virus, offering this unauthorized vaccine to relatives of the first confirmed cases.
This vaccine rVSVG-ZEBOV-GP, vsmade up of vesicular stomatitis virus (VSV), was developed by the Merck laboratory in 2016. Administered as a single dose, it has so far been shown to be successful in clinical trials on humans, but has not yet received marketing authorization (AMM). However, it was successfully tested in 2015 in Guinea. At the time, the Ebola epidemic in West Africa had killed more than 11,000 people. Published in 2017 in the journal The Lancet, the results of the vaccine in question give hope for an end to the epidemic in the DRC since of the 6,000 people vaccinated, including 200 children, none contracted the disease in the weeks following vaccination. On the other hand, the researchers admit that they do not know the duration of protection conferred by the vaccine, nor even its effectiveness in pregnant women, young children and people. immunocompromised.

Credit: screenshot / situation report n ° 8 / WHO – June 3, 2018
As pointed out World Health Organization (WHO) in its information bulletin of May 30, 420 people identified around four cases in Mbandaka have been vaccinated. “The vaccine has also been administered to health workers in the region.” Ring vaccination (the first people around a case) started in Bikaro on May 28 and continued “in the Iboko health zone”.
Other experimental treatments coming soon
According to a Tribune researchers Eric D’Ortenzio (Inserm) and Yazdan Yazdanpanah (Paris Diderot University), the WHO could soon offer other experimental treatments. More precisely, they are antiviral molecules and monoclonal antibodies (ZMapp, Remdesivir GS-5734, REGN3470-3471-3479, Favipiravir and mAb 114) which are still not approved. “Monoclonal antibodies work by targeting a protein present on the surface of the Ebola virus. By attaching to the virus present in the body of infected patients, they prevent the infection of new cells,” they explain. “If administered as quickly as possible,” these monoclonal antibodies could decrease “the expansion of the infectious agent, thus allowing time for the patient’s immune system to produce an effective response.”
For the moment, these experimental treatments “have demonstrated their effectiveness. in vitro, that is to say in an artificial environment such as a test tube or on animal models “, indicate the scientists. Despite this, the United Nations believe that in view of the epidemic context, they must be administered to all patients without being compared to a placebo Administering experimental treatments, the undesirable side effects of which are unknown, still raises a few questions. ethics: how do you know that you are not chasing the plague and replacing it with cholera?
.