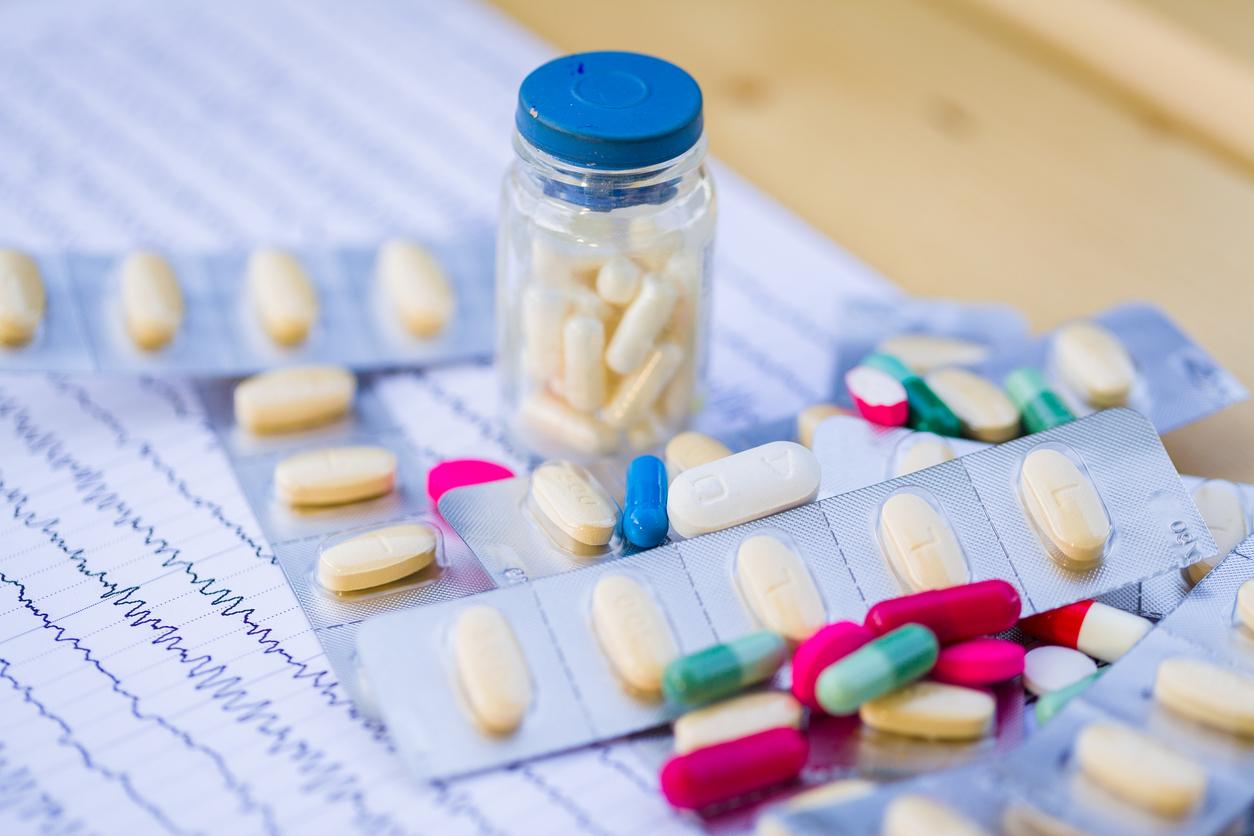
She never took Dépakine but was exposed to toxic emissions in the atmosphere from the factory producing the drug in Mourenx: a mother of two autistic children filed a complaint against X for endangering others.

- A mother of two autistic children suspects the Sanofi factory in Mourenx of being the cause of her children’s problems.
- This factory manufacturing Dépakine released unconventional levels of sodium valproate into the atmosphere until 2018.
- This active ingredient is known for its teratogenic effects: in the event of maternal exposure during pregnancy, it exposes the child to a high risk of malformations and neurodevelopmental disorders.
This is a new part that begins in one of the biggest French pharmaceutical scandals. According to information from our colleagues the newspaper The world, a mother of two children presenting neurodevelopmental disorders similar to those observed in children exposed in utero to Dépakine, filed a complaint against X for endangering others with the public health division of the Paris judicial court. This mother has never taken the drug but was exposed to air releases of sodium valproate (its active ingredient) at her workplace.
An antiepileptic drug responsible for malformations and neurodevelopmental disorders in thousands of children
Produced by the pharmaceutical giant Sanofi, this antiepileptic drug has only been banned for pregnant women or women of childbearing age since 2016. However, the first alerts were issued in the 1980s for the risks of congenital malformations and at the beginning of the 1980s. 90 for those concerning neurodevelopmental disorders. Over the period from 1967 to 2016, Dépakine is responsible for major congenital malformations in 2,150 to 4,100 children and neurodevelopmental disorders in 16,600 to 30,400 children, according to a study of the Medicines Agency (ANSM) and Health Insurance (CNAM) published in June 2018.
Sodium valproate: toxic releases into the atmosphere from the Mourenx factory
Located in Mourenx in the Pyrénées-Atlantiques, the Sanofi factory which manufactures Dépakine has already been singled out in 2018 for its unusual toxic discharges of sodium valproate and bromopropane (an organic compound classified as carcinogenic, mutagenic and reprotoxic). possible). “The annual flow is estimated at 13.4 tonnes/year according to the “realistic” scenario and at 20.2 tonnes/year according to the “envelope” scenario.” regarding sodium valproate, details the INERIS evaluation report (National Institute of Industrial Environment and Risks). The “realistic” scenario corresponds to the average flow measured, while the “envelope” scenario represents the maximum flow measured over this period.
Faced with this observation, the factory was ordered by the authorities to put an end to these emissions… But the damage has already been done for many local residents, like this mother of two children born in 2014 and 2016 and diagnosed autistic. Asked by The worldshe explains having carried out a Depakinemia test in 2018 which turned out to be positive even though the factory’s production was at a standstill.
Malformations, ASD: “this could represent thousands of children”
How many other victims of these toxic releases could there be in the region? “It’s difficult to quantifyexplains Marine Martin, president of Apesac (Association to Help Parents of Children Suffering from Anti-Convulsant Syndrome). But that could represent thousands of children.”The founder of the association wants investigations to be carried out among the population and in particular on children in the area. “As part of the investigation which began on November 10, I asked the investigating judge to take data from Health Insurance in order to see if among the inhabitants living in the Lacq basin [lieu où se trouve l’usine de Sanofi, ndlr]there would not be an abnormally high number of children with malformations and/or autism spectrum disorders”, she specifies before adding: “the cost would not be huge, so I hope she will accede to my requests”.

Contacted by AFP, the pharmaceutical company indicated that it had “not aware of this procedure”. Sanofi states that “at this stage, none of the studies already carried out or in progress”did not make it possible to highlight a particular risk” linked to “atmospheric emissions” generated by the manufacture of the drug.
Dépakine had already returned to the forefront last summer, following the publication of this study showing an increased risk of neurodevelopmental disorders in children whose fathers were treated with valproate or its derivatives (Dépakine, Dépakote, Dépamide, Micropakine and generics), in the three months before conception, compared to fathers treated with others antiepileptics (lamotrigine or levetiracetam).