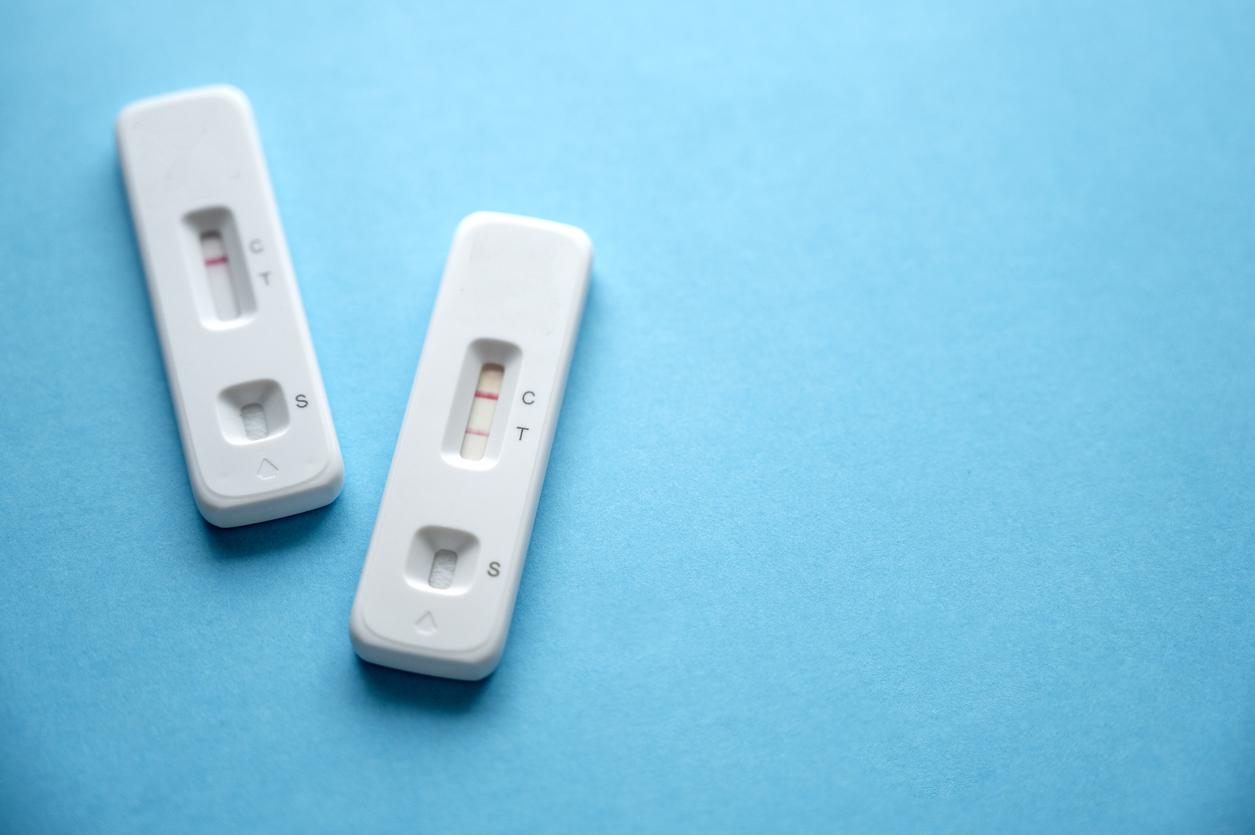
Antigen test: how does it work?
Called Elecsys SARS-CoV-2 Antigen, the test launched by the Swiss pharmaceutical company Roche can detect the presence of antigens specific to the disease. Like the PCR test, it is performed by nasal swab using a swab. The difference ? This antigenic test does not need to be analyzed in the laboratory. Simply put, it is much faster to get results. Indeed, the test is intended to be performed on machines (present in many hospitals and laboratories) which have the capacity to process up to 300 samples per hour. The results are given after 18 minutes (between 10 and 30 minutes maximum).
An alternative to PCR tests?
On Friday, October 9, the High Authority for Health (HAS) issued a favorable opinion on the use of these antigenic tests to screen the populations most at risk than the average. Among them, we can cite university students, slaughterhouse employees and nursing staff. As a reminder, on September 25, the HAS had already authorized these tests in a supervised manner to diagnose people who are symptomatic of Covid-19.
If the Haute Autorité de Santé ensures that they are extremely weak, it recalls that it is necessary to use them between the first and the fourth day after the onset of symptoms. According to the Swiss pharmaceutical group, the tests can be used as alternatives to PCR tests when these are not available or to help healthcare professionals screen symptomatic patients in the event that testing capacity is under pressure.
When is the launch?
The launch of the Elecsys SARS-CoV-2 Antigen test in large quantities is scheduled for the end of the year, in October, for countries accepting the CE standard (the European conformity mark). The Roche laboratory announced in a press release that it also plans to file an application for rapid approval (EUA) for the US market.