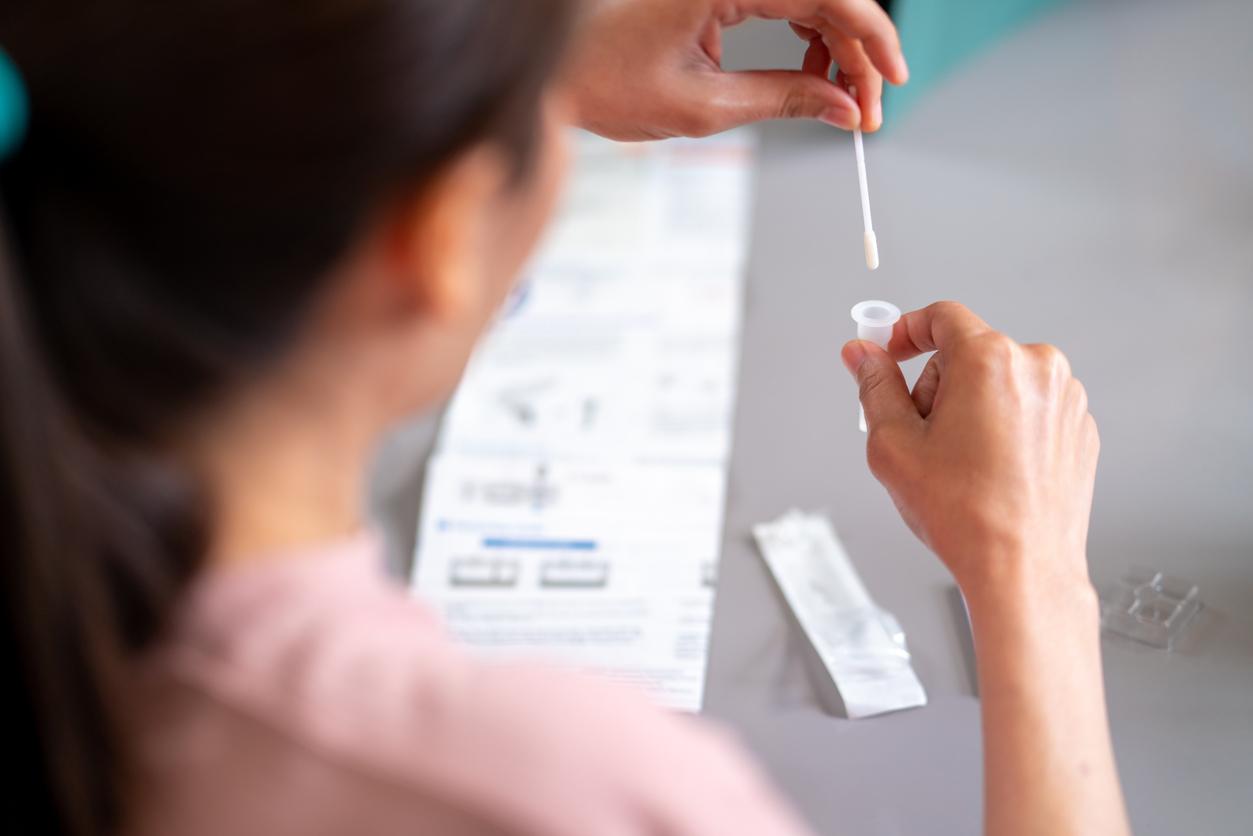
The self-tests, already validated by the High Authority for Health, will finally be deployed only from mid-April only in pharmacies and for a targeted public. And a new simple and non-invasive serological test has been developed and has a sensitivity of 90%.

- The self-tests will be deployed for young people over the age of 15, particularly at university, and populations far from care.
- They will only be available in pharmacies and the question of their reimbursement has not yet been decided.
- The new serological test, developed by Inserm researchers, is done by a simple prick of the fingertip.
To effectively fight Covid-19, it is crucial to know how and where the virus circulates. For this, several virus detection tests are available. Validated two weeks ago by the High Authority for Health (HAS), the self-tests were to complete the arsenal of tests alongside PCR, saliva and antigens. If a decree published in Official newspaper of March 27 authorizes their use, it will be necessary to wait, at best, for mid-April to see their deployment.
Deployment only in pharmacies for a targeted audience
The arrival of self-tests in France is taking place in small steps. Last Friday, the Ministry of Health announced during an online press briefing that this tool will be the subject of a “deployment supervised by the State”. Before their possible generalization, their sale can only take place in pharmacies. First, we must ensure that these tests are used “in good conditions”. For this reason, their sale in supermarkets “is not on the agenda“Unlike Germany where, since March 6, they have been available in supermarkets and have been very successful since many brands have reported stock shortages.
Not everyone will be able to buy the self-tests which will first be the subject of experimentation with “target audiences”. It concerns “audiences that we are not testing today”. In concrete terms, these are young people over the age of 15, while the HAS will soon reassess the advisability of extending their use to 10-15 year olds. The students and thepopulations far from care” such as precarious people or the inhabitants of certain overseas territories are mainly targeted. The exact list of these audiences is still being defined, specifies the ministry. The objective is to “to recover“positive cases”which would have gone unnoticed”, he adds.
The issue of reimbursement for this medical device is not settled either. “The strategy is not yet stabilized, particularly on the question of reimbursement according to the public, according to the age or the situation of the person”do we advance to the Ministry of Health
80% efficiency
These tests will be antigenic, that is to say capable of identifying the antigens produced by the immune system against the virus, while the PCR test directly detects the presence of SARS-CoV-2. The sample for the self-test will be taken through the nose, as for the other tests currently available, except that it will suffice to rub a cotton swab inside the nostrils, and no longer at the bottom of the nose. Then the cotton swab will need to be placed in a special solution, allowing the presence of antigens to be detected in less than half an hour. According to the existing models, the results are obtained either thanks to a strip on which the reagent is deposited, or via an application.
Self-tests are less effective than PCR and antigen but have shown initial encouraging results which, according to HAS, justify their deployment. They meet the criteria with a sensitivity of 80% in symptomatic people, 50% in asymptomatic people and a “specificity” – proportion of patients without disease having a negative test – of 99% in symptomatic people. The complete analysis of the performance of these nasal tests is still in progress and should take place in the coming weeks, following which the HAS could extend their use to 10-15 year olds.
A new serological test, simple and effective
At the same time, Inserm researchers, in collaboration with the British University of Oxford, have developed a new serological test to find out if anti-SARS-CoV-2 antibodies have been developed following a first infection. This test, say the researchers who presented it in an article published this Monday, March 29 in the journal NatureCommunicationsEast “reliable, inexpensive and requiring no specialized equipment”. It is based on “a unique reagent that causes agglutination of red blood cells in the presence of antibodies specific for the SARS-CoV-2 virus”. Tested on over 400 patient sera in the UK, it showed a sensitivity of 90%.
This method, known for more than 50 years, is used in particular to determine blood groups. “The secret of the simplicity of this test is based on the use of a single reagent which consists of a recombinant protein combining an antibody recognizing a molecule on the surface of red blood cells (glycophorin) with the RBD peptide of the Sars Spike protein -CoV-2 (the domain recognized by neutralizing antibodies against the virus)say the researchers. When brought into contact with blood, this reagent binds to red blood cells.” Blood can be taken by a simple prick from the fingertip, as people with diabetes do in particular to measure their blood sugar. “In addition, the supply of reagent is in lyophilized form requiring no refrigeration and the reading of the result is done with the naked eye.say the researchers. Another advantage: this reagent is easy to produce at low cost. With an estimated cost of 0.3 euro cents per test, it would therefore be affordable for countries with fewer resources..”

.