Vasoconstrictors, widely used to treat cold symptoms, present risks in the event of prolonged use or in combination between oral and nasal medications. The ANSM wants to reinforce the information on these products for doctors and patients.

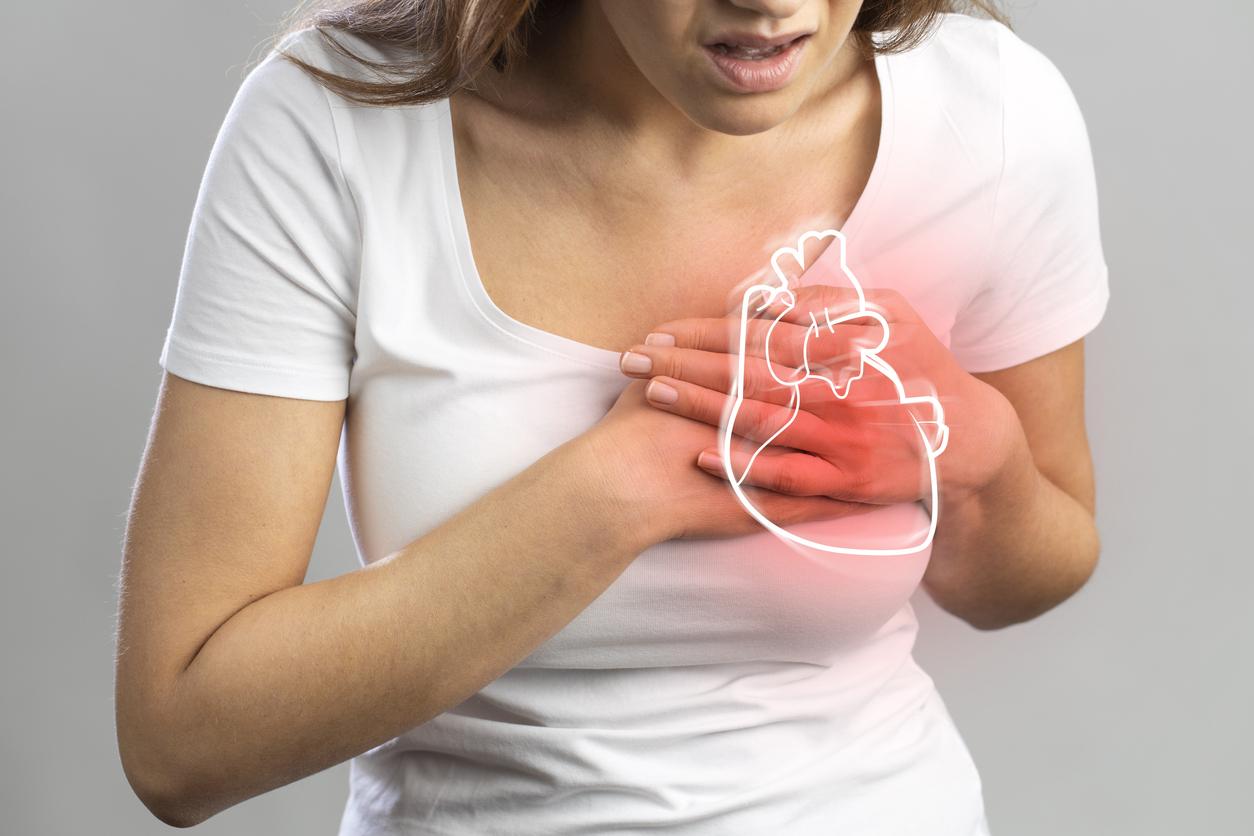
It is the first reflex in the event of a cold: to be delivered in pharmacy of the vasoconstrictor tablets – accessible without prescription for the products usable by oral route – which have the effect of unclogging the nose. But these drugs present risks of adverse effects that are certainly rare but serious. A recent pharmacovigilance survey presented to the National Medicines Safety Agency (ANSM) showed the persistence of these adverse effects during the use of these products, in particular the risk of myocardial infarction and ischemic vascular accident.
These risks recalled on December 17 by a press release from the ANSM are all the more important since vasoconstrictors are often the subject of misuse: treatments prolonged beyond 5 days or association between oral vasoconstrictors and nasally, not to mention the non-compliance with the contraindications appearing on the instructions for these drugs.
A dispensation aid form from January 2020
The ANSM has just called on all the players concerned, users, patients, healthcare professionals and manufacturers, to consider the implementation of measures aimed at preventing these adverse effects. From January 2020, an aid sheet for the dispensing of oral vasoconstrictors could be distributed to community pharmacists to remind them of the good uses of these drugs, the risks and advice for the treatment of colds. This sheet would be given to patients when these drugs are dispensed.
The affected products are: Actifed Cold tablets, Actifed Rhumle Day and Night tablets, Actifed LP Allergic Rhinitis tablets, Dolirhume Paracetamol and Pseudoephedrine 500mg tablets, Humex Cold tablets, Nurofen Cold tablets, Rhinadvil Cold Ibuprophene/Pseudoephedrine tablets coated and in soft capsule 200mg, Rhinureflex in film-coated tablet and Rhumagrip in tablet.

.