July 26, 2017
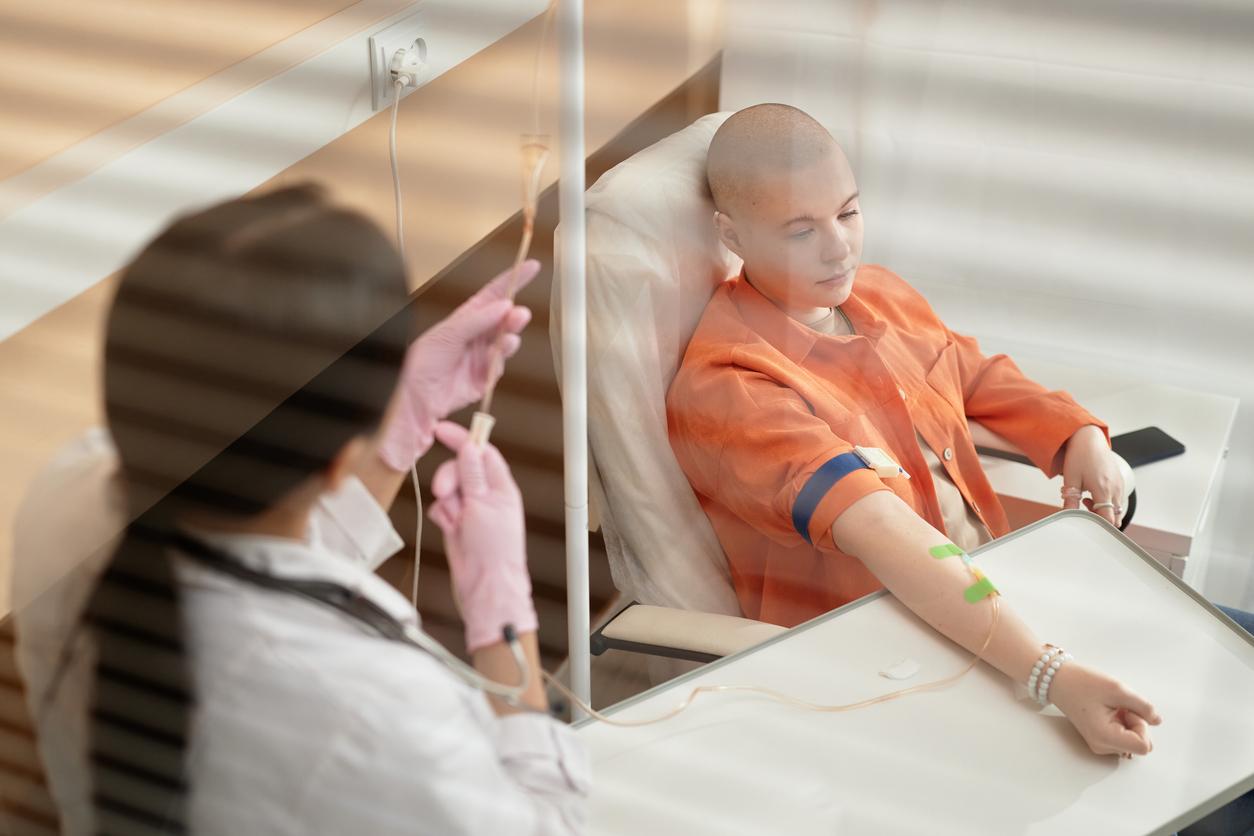
Cancer treatments could be revolutionized by the American group of pharmaceutical companies Norvatis. On July 12, 2017, the Food and Drug Administration (FDA) advisory committee issued a favorable opinion for the marketing of an individualized therapy against leukemia.
Individualized treatment for leukemia
July 12, 2017 the Food and Drug Administration (FDA) advisory committee has issued a favorable opinion on the marketing of an individualized treatment for leukemia cancer. This treatment is produced by the group of American pharmaceutical laboratories Norvatis. The final FDA opinion will not be delivered until October 3, but this news could revolutionize the care of patients with blood cancer.
The drug (CTL019) developed in these pharmaceutical laboratories is composed of a process dosed according to each patient. Intended to restore the capacities of the immune system of the sick person, this treatment would allow “ reprogram directly “ the patient’s cells to fight cancer.
82% of beneficiaries of individualized treatment are in remission phase
For pharmaceutical companies used to mass production of treatments, individualizing drugs is a costly challenge. On the other hand, it seems that the game is worth the candle. The therapeutic experiments carried out by Norvatis over the past five years have in fact made it possible to treat 400 patients. According to THEechoes, some of them are “ almost cured “ and can lead a normal life again.
In addition, 82% of beneficiaries of this individualized drug were in remission after three months of treatment, whereas they previously had treatment failures. If the scientific community does not yet fully understand the process, around fifteen pharmaceutical companies are currently working on the issue. Individualized treatments against blood cancers could therefore very soon change the way of approaching these diseases.
Read also: Leukemia: a change in diet could help
Marie-Hélène Hérouart