The tests of the artificial heart will resume. 20 patients will soon be implanted, announces Carmat, which manufactures the device.
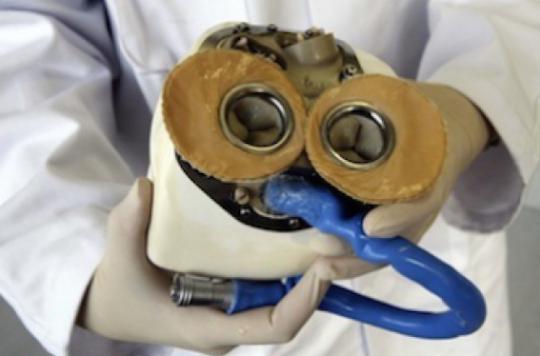
The artificial heart starts beating again. The Carmat company, which produces the medical device, announced on July 13 the imminent resumption of clinical trials. Almost 7 months elapsed between this declaration and the death of the last implanted patient. This time, less fragile patients can be recruited.
The total artificial heart developed by Carmat is intended for patients with terminal biventricular heart failure. It completely replaces the organ in its various functions in patients whose two ventricles are not working properly.
A first wave of tests was carried out to determine the feasibility of this core. Four patients who have since died have far exceeded the team’s hopes: success was subject to a one-month survival. It varied from this interval to 9 months. All were at the terminal stage of their disease.
20 volunteers
This time around twenty volunteers will be able to benefit from the system developed in France. In addition to people with end-stage disease, others who are waiting for a heart transplant and have a low chance of getting it will be eligible. The Personal Protection Committee (CPP) and the National Medicines Safety Agency (ANSM) have agreed to a pivotal study in the country. “The favorable opinion of the CPP, as well as the authorization of the ANSM, confirm the validity of the research objectives and the expected conclusions”, welcomes Dr Piet Jansen, medical director of Carmat.
The company also announced its intention to expand overseas. The company is taking steps to establish patients in other European countries. “The results of studies in France and the rest of Europe will be integrated into the clinical module of the dossier, the assessment of which has been entrusted to DEKRA”, explains Marcello Conviti, Managing Director of Carmat. This company provides certification services for CE marking.

.