The blood test, based on analysis of microRNA sequences, was able to diagnose amyotrophic lateral sclerosis with up to 98% accuracy, according to a study.
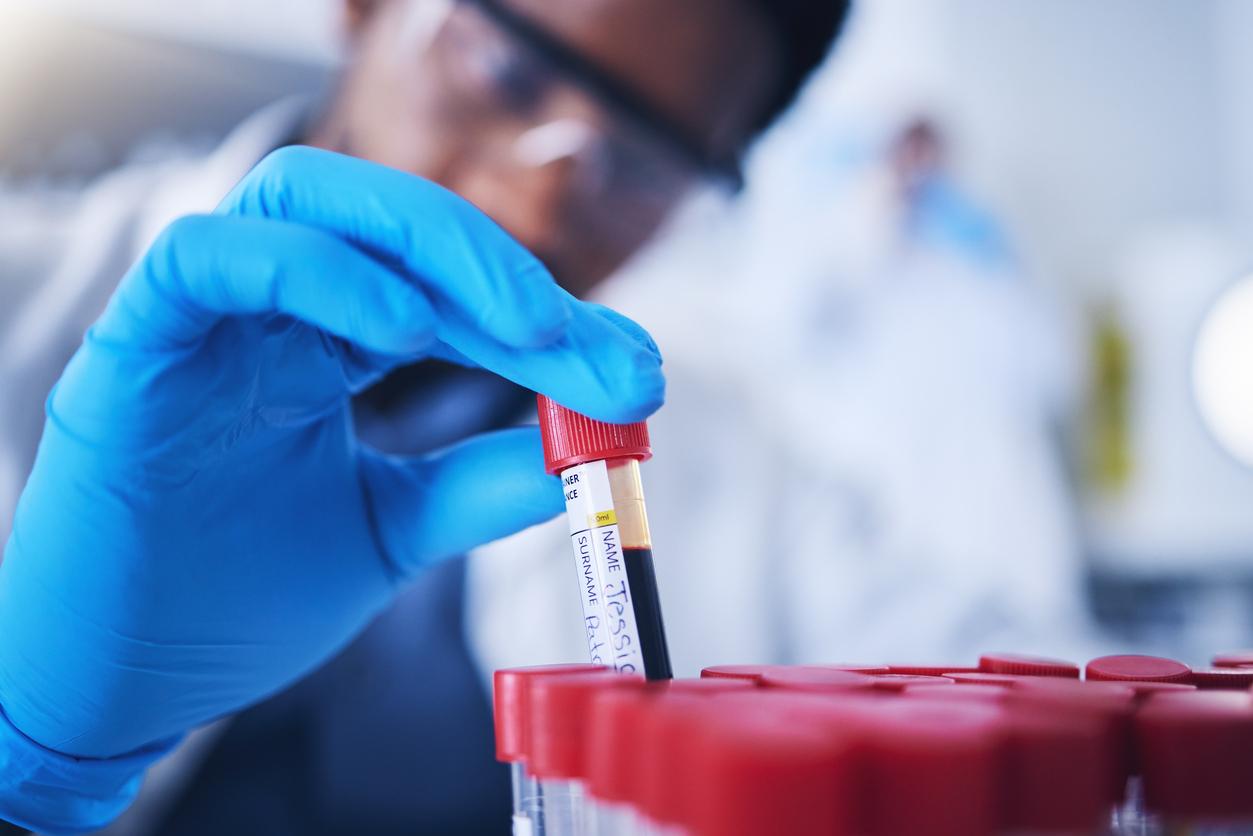
- The blood test requires only a simple blood draw. It is based on small nucleic acid sequences, known as microRNAs.
- Analysis of microRNA sequences from samples from 471 patients made it possible to develop a unique “ALS fingerprint” and distinguish between blood samples from ALS patients and healthy controls.
- “A timely diagnosis will allow treatment to begin earlier, leading to better outcomes for affected patients.”
This could be a small revolution in screening. A team of scientists from Brain Chemistry Labs, a research institute in the United States, has developed a blood test that can accurately and, above all, more quickly diagnose amyotrophic lateral sclerosis (ALS), better known as Lou Gehrig’s disease. The results of the study were published in the journal Brain Communications.
A blood test based on microRNA sequences
A rare disease affecting around 8,000 people in France, ALS is a neurodegenerative condition that affects the neurons of the brain and spinal cord, leading to muscle atrophy and progressive paralysis until death, generally within two to five years. “If the diagnosis is based on a thorough clinical examination, it can take up to twelve months to be definitive, during which time the condition of patients can deteriorate considerably, explain the researchers in a press release. Not to mention that diagnostic errors occur in no less than 68% of cases, which further complicates treatment.”
Hence the crucial interest in this new screening test. The blood test, which requires only a simple blood sample, is based on the analysis of small sequences of nucleic acids, known as microRNAs, extracted from tiny vesicles released by the brain and nervous system.

Diagnose quickly to treat earlier
Analysis of microRNA sequences, carried out from samples from 471 patients suffering from the neurodegenerative disease, allowed scientists to develop a “SLA fingerprint” unique comprising eight distinct microRNA sequences. “These sequences can differentiate, sensitively and specifically, between blood samples from ALS patients and samples from healthy controls or patients with conditions that mimic early-stage ALS, with an overall accuracy of up to 98 percent.”
Researchers hope that this blood test, now validated by four separate trials, will become a commonly used tool by neurologists to screen for ALS. “A timely diagnosis will allow treatment to begin earlier, leading to better outcomes for affected patients.” While the procedure is not yet ready for routine medical use, they expect the test to be available within 18 to 24 months.