As part of the ongoing revolution in cancer treatment, a new pathway for boosting immunity against cancer has just taken a crucial step towards specific treatments. A clear breakthrough.
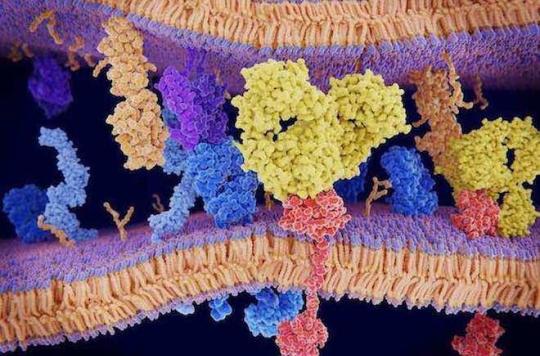
Cancer defends itself against the immune system, which normally protects the body by disrupting its functioning. One of the methods used by cancer cells is to activate the PD-1/PD-L1 system on the surface of specialized white blood cells, T lymphocytes, to slow them down and camouflage themselves in a way. This is what is currently used by anti-PD-1/PD-L1 immunotherapies, immunotherapies that are revolutionizing the treatment of many cancers.
Another method of braking the immune system by cancer has just been discovered by a team of Belgian researchers from the University of Louvain. They elucidated the three-dimensional structure of an assembly of proteins on the surface of Treg lymphocytes, immune cells that are normally responsible for curbing immune responses. They also discovered how an antibody could best block this assembly and the immunosuppression it induces. Such an antibody could serve to stimulate immunity against tumor cells in cancer patients. The study is published in the journal Science.
Immunosuppression and cascade of interactions
Among white blood cells, Treg lymphocytes (regulatory T lymphocytes) are immunosuppressive cells that normally counterbalance excessive immune reactions, in order to prevent the onset of autoimmune diseases. But in cancer patients, they play a deleterious role by moderating immune reactions against tumor cells.
Treg cells mediate their effects by producing a messenger protein, called TGF-beta. This messenger transmits inhibitory signals to nearby immune cells, including those thought to destroy tumors in cancer patients.
Block the release of TGF-beta
The way Treg cells produce TGF-beta is complex and finely regulated, as it is very potent and must be kept under strict control. Three years ago, Professor Sophie Lucas and her team at the University of Louvain discovered that TGF-beta is released by Treg lymphocytes from a protein called GARP, present on the surface of Tregs. His team also discovered that it was possible to block the release of TGF-beta by GARP using specific antibodies, but the most effective ones had to be found.
Molecular mechanisms elucidated
The researchers used X-ray crystallography to study the three-dimensional structure of GARP and TGF-beta molecules as they produce their inhibitory effects. They then realized that GARP looked like a horseshoe overlapped by TGF-beta. The two molecules are so tightly assembled that TGF-beta itself contributes to the horseshoe structure of GARP. If we put a specific antibody, it will stick the two molecules to each other, while blocking the small active part of TGF-beta which will not then be released, and will therefore be prevented from transmitting its inhibitory message to other immune cells.
The visualization of this large molecular assembly is a key step in the feasibility of blocking the activity of TGF-beta emanating from Treg lymphocytes. This breakthrough leads to extremely specific approaches to treat various diseases associated with altered activity of TGF-beta or Treg cells, in particular for cancer immunotherapy.
.