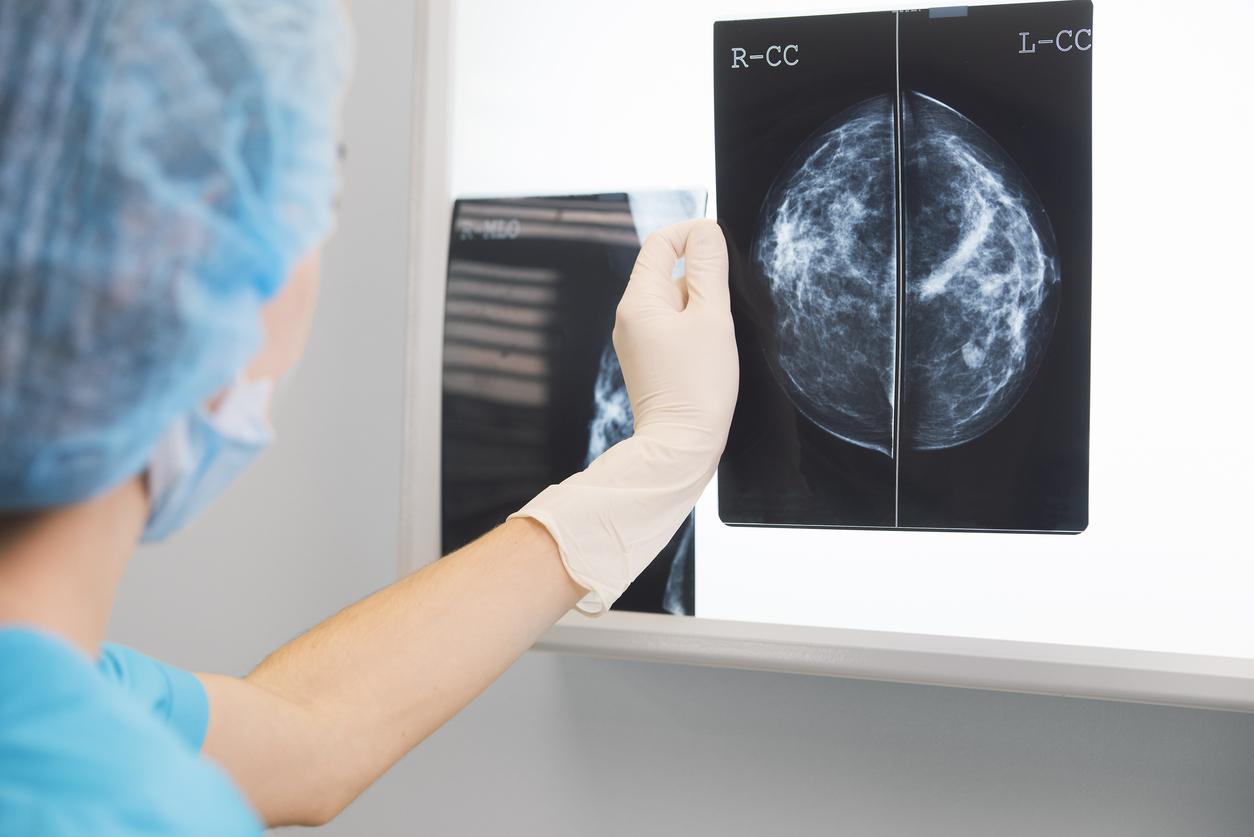
Talimogen laherparepvec oncolytic virus (TVEC) therapy, combined with standard chemotherapy, has been shown to be effective against triple-negative breast cancer, according to the results of a phase 2 clinical trial.
- Many breast cancer cells have estrogen or progesterone receptors. They may also have receptors for a protein called HER2, or ErbB2.
- Triple-negative breast cancer is made up of cells that don’t have any of these receptors.
- Due to the absence of these receptors, triple negative breast cancer is considered a separate type of breast cancer with its own treatment options.
Triple-negative breast cancer accounts for about 15% of all breast cancer cases. Patients with this subtype of breast cancer generally have poorer outcomes compared to other breast cancers, hence the need for improved treatments. A new therapy is being studied at the Moffitt Cancer Center. It involves a certain type of virus that infects and kills cancer cells.
In a new article published online February 9, 2023 in the magazine NatureMedicineresearchers share results from a phase 2 clinical trial of this virus, called talimogene laherparepvec oncolytic virus, combined with standard chemotherapy in patients with early-stage triple-negative breast cancer.
How does the oncolytic virus help destroy cancer cells?
The virus in question is an attenuated herpes simplex virus 1 (HSV-1) which includes coding sequences for a protein, called GM-CSF protein, capable of stimulating the immune system. It is injected directly into the tumor and undergoes replication in the tumor cells, leading to their degradation and the production of antigens specially dedicated to fighting the tumor. Immune cells can recognize antigens, infiltrate the tumor and target cancer cells to destroy them. Additionally, the GM-CSF protein made by the virus helps call for reinforcements in the battle against the tumor by acting as a beacon that guides immune cells to it.
Talimogen laherparepvec oncolytic virus (TVEC) therapy is approved for the treatment of advanced melanoma. The Moffitt researchers therefore wanted to assess whether it could also be effective in combination with standard chemotherapy when the virus was given to patients with triple-negative breast cancer before surgery.
45% of patients achieved treatment response
In a phase 2 trial involving 37 patients, 45.9% achieved a response, 89% of patients remained healthy two years after treatment, and no recurrences occurred in patients with a strong response. The safety profile did not differ significantly from what was expected from standard chemotherapy except for higher levels of mild fever, chills, headache, and pain at the injection site.
“Our results demonstrate that TVEC, when added to systemic chemotherapy, can augment responses in high-risk, early-stage triple-negative breast cancer. There is evidence for robust immune activation in the tumor”said the medical director of the office of clinical trials at the Moffitt Cancer Center and lead study author, Mr. Soliman, in a communicated.