Leem wants to facilitate and accelerate access to innovative molecules that could treat rare diseases. He calls for a dedicated 3rd National Plan.
Drug manufacturers want stronger action against rare diseases. 2016 marks the last year of the second National Rare Disease Plan, which began in 2011. On the occasion of a workshop on innovation in this area, the pharmaceutical companies (Leem) called for a third plan. The union has issued 20 proposals in this direction, broken down into four axes.
The first is to develop research around rare diseases. For industries, this means strengthening public / private partnerships, more links between research teams but also a pooling of work tools as well as registers and databases.
Promote the innovative character
The second axis advocated by Leem directly involves patients suffering from these pathologies, 3 million in France. The union believes that the development and availability of innovative molecules should be encouraged. A requirement that goes through several stages, particularly financial. He therefore proposes to promote the innovative nature of the drugs being tested, taking into account their impact on the quality of life of diseases.
The “financial risk (is) important for the laboratory” which develops treatments for rare diseases, specifies Leem. Indeed, 7,000 have been identified to date, but most of the time they hit a very small target. In two thirds of cases, only 0 to 5 patients are affected by the pathology. It is undoubtedly a niche market but which does not benefit from the appropriate measures. Thus, currently less than 5% of identified rare diseases benefit from a treatment approved by the health authorities.
Simplify and relax
To compensate for this risk taking, funding and disease management need help, says the union. In the field, this translates into joint work between patients, public authorities and manufacturers when setting prices, and changes in contracts with health authorities. The aim is simplification and more flexibility.
“France, which was a pioneer in the field of rare diseases, must keep its European leadership and be at the forefront of innovation, by ensuring that it pushes forward thinking and decisions about taking into account the specificities linked to this mosaic of diseases in an increasingly constrained budgetary context ”, considers Philippe Lamoureux, general manager of Leem in a press release.
The last point is not the least: strengthen cooperation between those involved in the management of rare diseases. Leem therefore calls for more space in the evaluation of the second rare disease plan, and wishes to amplify France’s voice in the European plan. Patients are not absent from the union’s requests: they must be more involved in evaluating the therapeutic value of innovative molecules.
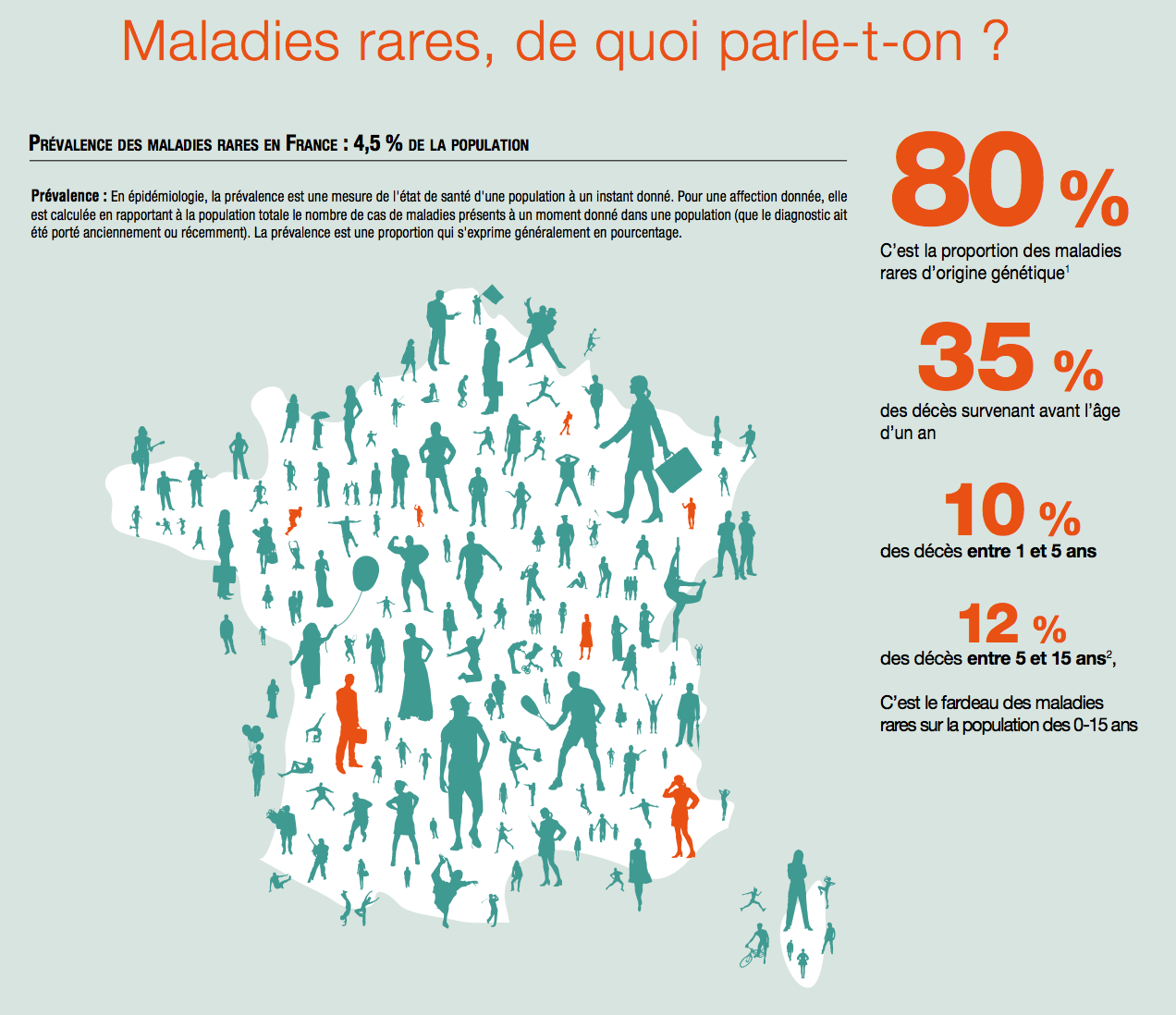
Source : The Leem
.