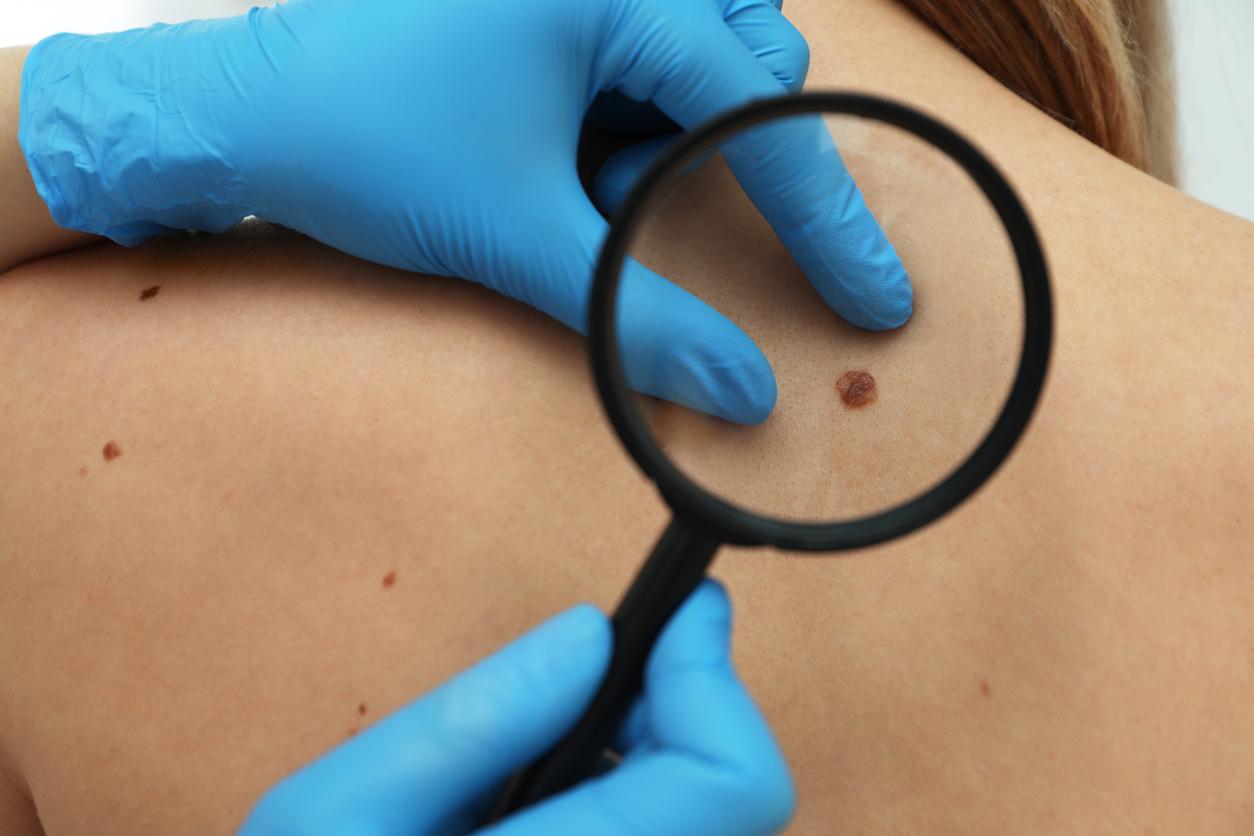
The search for treatments against virulent forms of melanoma seems promising. Three recent studies highlight the benefits of immunotherapy.

Researchers are enthusiastic about melanoma immunotherapy. Several studies are presented at the annual congress of theAmerican Society of Clinical Oncology (ASCO) held in Chicago (Illinois, USA). Two antibodies are particularly attracting attention: nivolumab and ipilumab, which target and block the “gatekeepers” of T cells (PD-1 and CTLA-4). These drugs disarm the defenses of tumors against the immune system, while boosting the latter’s ability to fight melanoma, a serious tumor of the skin.
Serious side effects
One point in common between the three studies presented at the ASCO congress: promising advances in the survival of patients after serious melanoma. Ipilimumab, already approved by the American health authority, the FDA, for the treatment of inoperable metastatic melanoma (stage 4), is also beneficial against stage 3 melanoma at high risk of recurrence. The risk of recurrence drops by 25% in treated patients and relapse-free survival climbs to 46.5% compared to 34% with placebo. “This trial on ipilimumab is the first to show that it is possible to prescribe these drugs earlier in the course of the disease, when they are more beneficial and can cure more patients,” says Professor Alexander with satisfaction. Eggermont, Director General of the Gustave-Roussy Cancer Institute (Villejuif, Val-de-Marne). The side effects of such treatment, however, are considerable: 5 of the 234 patients treated died as a result of the treatment, and half of the participants stopped taking their medication.
Benefits against metasatic forms
The second promising drug, nivolumab, is also of interest to doctors. In a phase 1 trial conducted by the David-Geffen School of Medicine at the University of California, Los Angeles, 221 patients previously treated with ipilimumab for metastatic melanoma tested the drug. At one year, survival is 69% and 88% of melanomas have responded to treatment. “We are excited to see that MK-3475 is effective in previously untreated patients as well as those who have received multiple treatments, including ipilimumab,” enthuses Antoni Ribas, co-author of the study. “This is early data, but it highlights that we are facing something really important. “
An effective combination
But it is the combination of the two antibodies that arouses the most interest. A phase 1 trial evaluated the efficacy of an ipilimumab-nivolumab combination in 53 patients with advanced inoperable melanoma. For 4 cycles, the patients took a combination of the two molecules every 3 weeks and then nivolumab alone according to the same principle. At six months of treatment, patients whose cancer was no longer progressing and those with moderate side effects continued the combination treatment every 12 weeks for 8 cycles. The combination of the two antibodies doubled the median survival compared to a single molecule. 7% of patients even presented complete remission. “A few years ago the median survival of patients with advanced melanoma was no more than one year, and only 20-25% survived two years, so it’s really remarkable that we are seeing a median survival of more than three years in this. study ”, underlines Mario Sznol, principal author of the study. It remains to confirm the benefits of these treatments in larger trials.
.