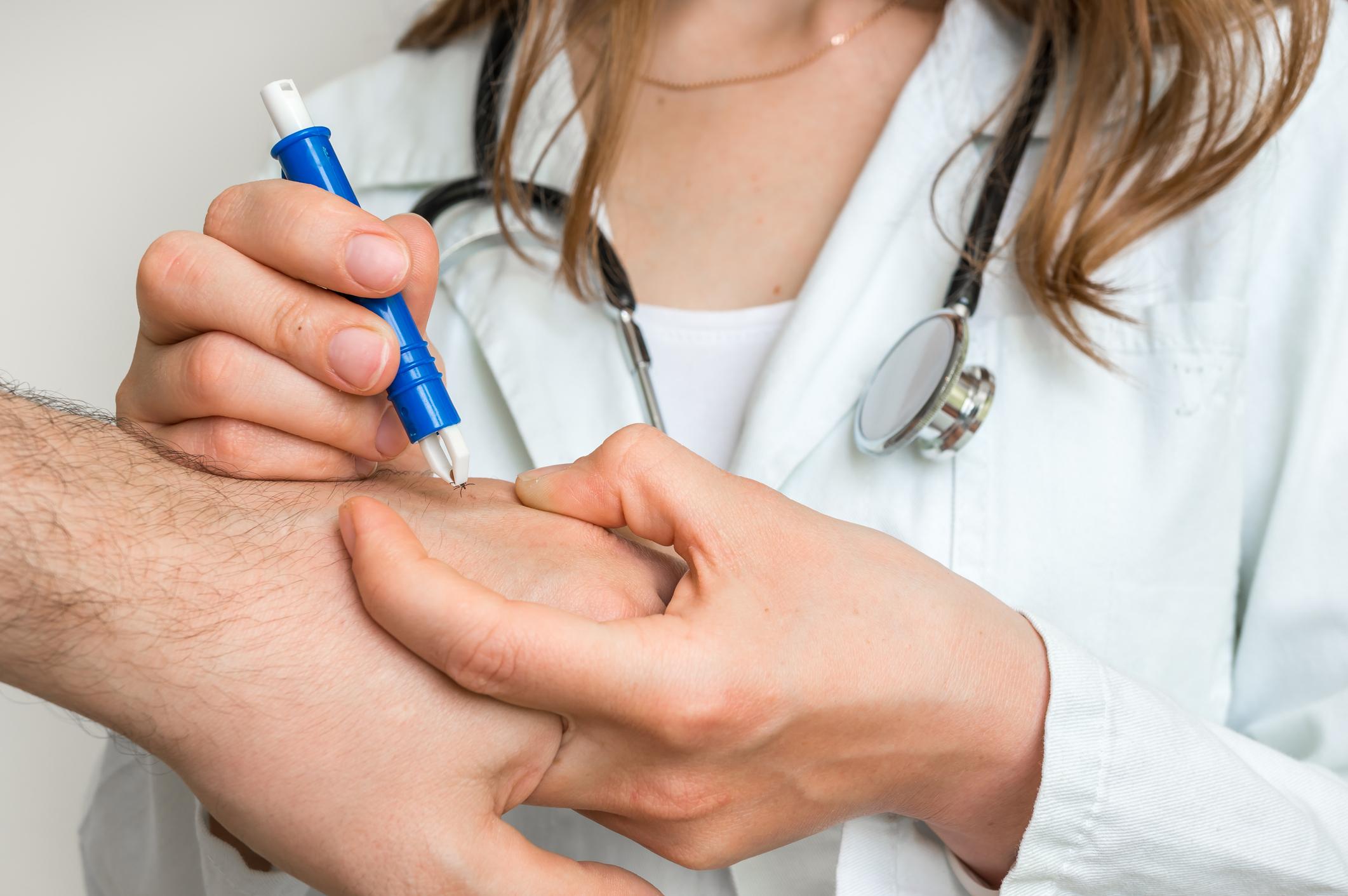
130 Lyme patients are going to assign civil liability to two laboratories which they accuse of marketing tests which have not made it possible to diagnose them.
Boosted by the recent announcement of the national plan to combat tick-borne diseases, Lyme patients are galvanized. On Tuesday, October 18, 130 plaintiffs will engage the responsibility of two pharmaceutical companies. They accuse the latter of marketing tests that have not made it possible to diagnose them.
In a press release signed by lawyers Me Catherine Faivre and Me Julien Fouray, these people affirm that these tests “by ignoring the sick and the disease have proven their total ineffectiveness”. “It is time to put an end to this health scandal”, they chant. And for them, the responsibilities do not stop with the industrialists, “the health authorities, the State and the Minister of Health in a personal capacity will also have to be placed in front of their responsibilities”, they add.
A grouped action
It’swithin the framework of the LYMACTION defense and appeal unit, which today brings together half a thousand complainants, that this first wave of summons for compensation before the tribunals de grande instance (TGI) of Paris and Nanterre will be launched. “Remedies that announce others”, already warn these patients. More specifically, it is the French laboratory BioMérieux and the Italian biotechnology company DiaSorin who are targeted here. Both market the incriminated test called “Elisa”.
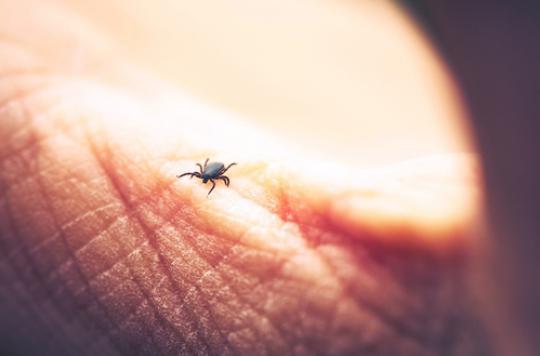
It is the only one currently authorized in France to detect Lyme borreliosis, a disease transmitted by ticks.
To Agence France Presse (AFP), the two lawyers specified that they were going to carry out “a grouped action” and not a “group action”, possible since the publication of a decree last month, but which “we is closed, as it is subject to very restrictive conditions”. And according to Me Julien Fouray, civil liability actions should in the future be brought against other laboratories that have produced the “Elisa” test, in particular before the Strasbourg TGI.
Screening decried
Based on a blood sample, the “Elisa” type tests result from a care consensus established by the health authorities in 2006, on the basis of American directives valid for American strains transmitted by ticks. Criticized for a long time, these tests are even now questioned by the government since the latter has recognized in its plan against Lyme disease the need to develop new diagnostic tests.
As a reminder, Lyme disease is transmitted to humans only by tick bite. Human contaminations are more frequent during the period of maximum tick activity, in France between the beginning of spring and the end of autumn. In 2014, the number of new cases in France was estimated by the Sentinel Network at 26,146; this figure has been stable since 2009, recently indicated the Ministry of Health.
Find our first supplement
broadcast September 30, 2016 on the WhyDoctor website.
It was devoted to Lyme disease
with interviews with specialists,
patient testimonials and practical information. 
Find the program L’invité Santé
with Pr Christian Perronne (Garches Hospital)
aired 05/12/2016
.