The French company Carmat announces that it has carried out an artificial heart implantation abroad. A first in Kasakhstan for this company, a choice that raises many questions.
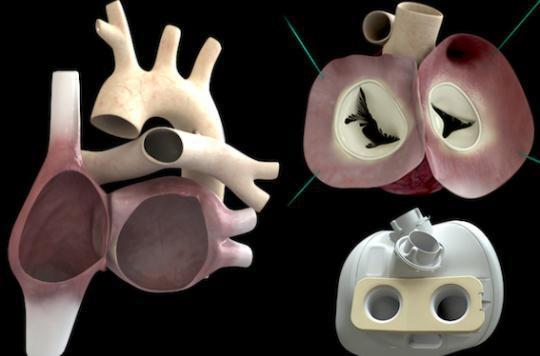
This is the first implantation of an artificial heart carried out abroad by Carmat according to the press release of this company. The French company had until now implanted its heart only in France. The operation took place in Kazakhstan, at the National Research Center for Cardiac Surgery, located in Astana, the Kazakh capital.
The company had carried out in 2013, the first permanent implantation of an artificial heart, before that they were intended only for patients awaiting transplant. The first patient died 75 days after the operation. The four implantations that followed also ended in the death of the patients. The National Medicines Safety Agency then asked the French company for new guarantees. Testing was able to resume last May.
Go abroad, to escape the weight of French regulations?
The choice to carry out these tests in Kazakhstan raises many questions. Why not do these tests in France? Is it linked to too strong a regulatory constraint? “It is true that today the validation stage by the National Medicines Safety Agency is complicated. The agency was traumatized by a series of problems: growth hormones, levothyrox, the Mediator. There is, on his part, today, a great reluctance, in any case a very great caution, before giving an authorization ”, explains a cardiac surgeon. It takes several months for the ANSM to provide an opinion.
The weight of the precautionary principle
Abroad, validation is much faster, it only takes a week in the UK for example. “There are European regulations, but in France, other rules have been added,” adds this surgeon. “We must not allow anything and everything, that’s for sure. But we feel a form of withdrawal from the ANSM, and we pay all the consequences ”. To the point that some choose to go abroad, like Carmat. “It is a phenomenon that exists and, if it continues, it will increase,” said this surgeon.
The Carmat company specifies in its press release the conditions for carrying out this test: “the first installation […] Has been done […] in compliance with the protocol of the pivotal study approved by the National Agency for the Safety of Medicines and Health Products (ANSM) and in accordance with local authorizations. “
But why Kazakhstan?
Carmat did not wish to answer our questions on the specific choice of Kazakhstan. This former Soviet country is widely criticized today for its poor respect for human rights. Amnesty International denounces the widespread use of torture in the justice system, the recurrent use of police violence. As for Reporter Without Borders, the association considers that press freedom is in a “difficult” situation. Another aspect to consider is the country’s great oil wealth.
In its press release published yesterday, Carmat announced the opening of new centers to carry out other establishments. Without specifying more about their location. And the stock price soared.
.