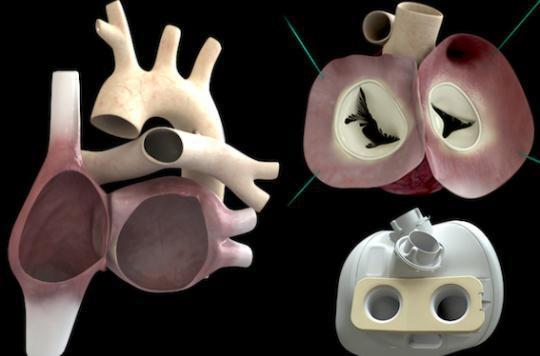
Determined but angry. This is how the boss of Carmat, promoter of the artificial heart, appears today in the columns of the Parisian. Since the death of the fifth patient last October, Stéphane Piat and his fifty collaborators have been waiting for the green light from the National Medicines Safety Agency (ANSM) to restart the implantations as part of the clinical trial.
If the ANSM proves this situation of “ stand by in everyday life, it’s to ensure patient safety, she says. The last lived only a few weeks with the prosthesis.
Yes, but “the death of the patient has nothing to do with the prosthesis”, objects Stéphane Piat, who recalls that the candidates for the artificial heart are at the end of their course. “There were problems; there will be, he pleads. But our prosthesis works. Patients have lived for months thanks to her”.
Behind this battle of arguments, there are two opposing conceptions of innovation. “The ANSM observes the precautionary principle”, explains the boss of the French company. But this principle is outdated in many countries where we speak rather of benefit/risk”, he continues.
Listen to the interview with Stéphane Piat
December 8, 2016
A dialogue of the deaf that could lead the company to set up abroad? The risk exists. “We are ready to go again. But I wonder, answers Stéphane Piat. Do we really want to do innovation in France? »
The United States, for example? The FDA, the US health authority, he admits, has “a more pragmatic approach”. One thing is certain. Even if the trial restarts in France, the company will refuse to continue at the rate of two trials per year.
The company’s share today stands at 29.44 euros, four times less than at the start of the trials in 2013, recalls the journalist from the Parisian Florence Mereo.