Bayer has announced the end of marketing of the Essure implant in all European countries. It remains available in the United States, where a class action is underway.

Officially, this is not for security reasons, but for a “commercial” reason. The Bayer laboratory said on Monday that it will no longer place Essure sterilization implants on the European market. The device will only be available in the United States, where group action by patients complaining of side effects is also underway.
The pharmaceutical group announced the end of the marketing of the implant on Monday in a press release. This decision “extends the one announced at the end of May to stop making Essure available in most countries”.
Marketing suspended in August
“Consequently, Bayer will not resume the marketing of Essure in France and will not continue the process of renewing the CE marking of Essure for European countries, Iceland, Liechtenstein and Norway,” the statement said.
Indeed, the marketing of Essure implants had been suspended at the beginning of August for three months in the European Union. The body responsible for renewing their certification had requested additional information on this definitive contraceptive device.
“As a precautionary measure, the ANSM then asked Bayer Pharma AG to recall the products in stock and asked to no longer implant the Essure medical device”, indicates the National Medicines Safety Agency in a press release. .
Side effects
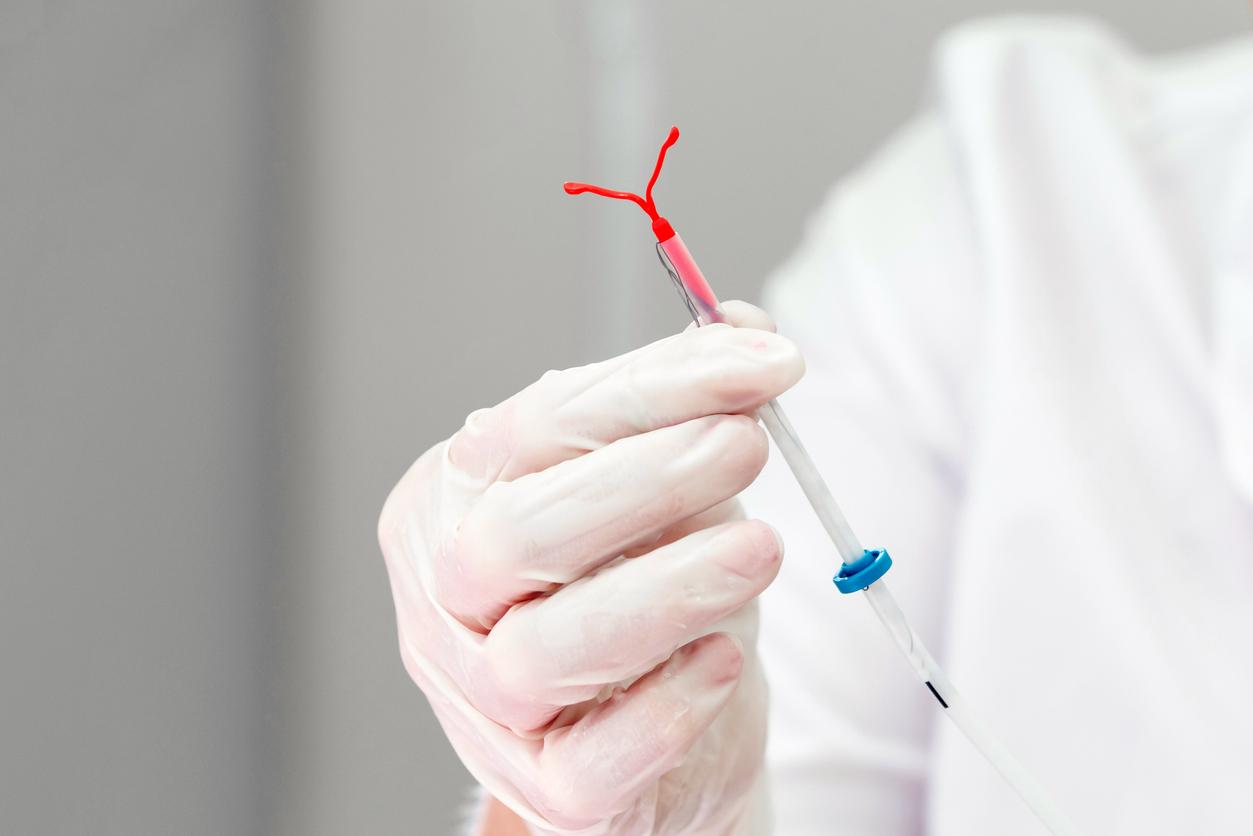
These small spring-shaped female sterilization implants are disputed by some women, especially in France and the United States, because of sometimes serious adverse effects occurring after their implantation.
“The victims of the Essure device can be delighted that it has been definitively withdrawn from the market in France. This solution corresponds to what the victims that I represent have been asking for a long time, ”reacted in a press release Me Charles Joseph-Oudin, lawyer representing several hundred French patients.
For its part, the ANSM “wishes to reassure women carrying the Essure implant on the favorable risk-benefit ratio of the device”, explains the Agency in its press release. This benefit-risk ratio “was evaluated last April by a committee of experts meeting at the Agency on the basis of data from the literature, surveillance and the results of the epidemiological study, covering more than 100,000 women, ”she says.
Consult your doctor
For women who have no symptoms, “who represent the vast majority of women with the Essure implant, there is no argument to date to advise the removal of the device,” says the ANSM. For others, who have felt symptoms, the Agency recommends seeing a doctor “so as not to overlook an underlying pathology”. In the absence of such a diagnosis, the interest of a withdrawal can be considered between the woman concerned and the doctor, she adds.
A meeting on these subjects will be held “very soon” at the Ministry of Solidarity and Health with representatives of associations, health professionals and the various institutional actors concerned, we can still read.
.