The American health authorities have just authorized “as a matter of urgency” the transfusion of blood plasma from people cured of Covid-19 to hospitalized patients in critical condition. What is this controversial treatment?
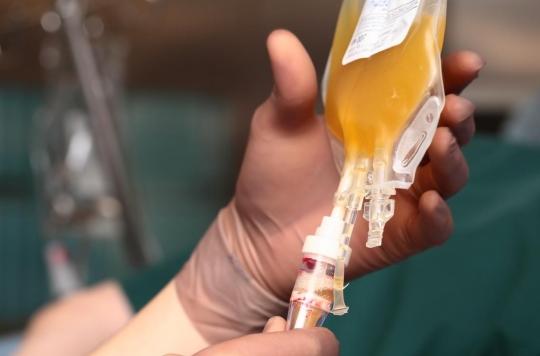
- Blood plasma treatment has been known and used since the 19th century. It consists of boosting the immune system of patients by injecting them with blood plasma from patients cured of Covid-19.
- Its effectiveness is hotly debated within the scientific community.
- The World Health Organization considers current evidence of its effectiveness to be of “low quality”.
A “historic breakthrough” who could “save a considerable number of lives”. This is how US President Donald Trump on Sunday August 23 described the decision of the Food and Drug Administration (FDA), the United States health agency, to transfuse patients hospitalized in serious condition with blood plasma from people who have recovered. of Covid-19.
Authorized “emergency” and on a large scale by the FDA, this treatment is nevertheless the subject of discussions within the scientific community, and has not yet definitively proven its effectiveness.
What is plasma transfusion therapy?
Used for the first time in 1892 against the bacillus responsible for diphtheria, then in 1918 to fight the Spanish flu, this method consists of administering to Covid-19 patients blood plasma from patients who have recovered from the disease. Considered as “liquid gold” by some scientists, blood plasma contains a large number of antibodies which, injected in large numbers, would allow the patient to develop immune defenses able to fight the SARS-CoV-2 virus.
Is the treatment effective?
Already authorized in other countries, such as France, Austria or China, treatment with blood plasma is no less controversial. In question: its effectiveness difficult to prove. Asked by France 24epidemiologist at the Karolinska Institute in Stockholm Joakim Dillner explains: “Clinical research and hospital use indicate, indeed, that blood plasma transfusions can reduce hospitalization time and lower mortality rates.”
However, for the time being, clinical trials attesting to its effectiveness are still lacking. A Mayo Clinic study (United States) has for example shown that early blood plasma transfusions reduce the mortality rate in patients. But the study is not a clinical trial and has not yet been peer-reviewed.
Other work, this time carried out by Johns Hopkins University (United States) aims to immunize subjects to SARS-CoV-2 by injecting them with plasma from former patients. The final results are not yet known.
The FDA itself recalled in a statement that this treatment is currently used on more than 70,000 patients, either in critical condition or in clinical trials. “I am committed to bringing safe and potentially useful treatments for Covid-19 to market as quickly as possible to save lives.said Dr. Stephen M. HahnCommissioner of the FDA. We are encouraged by the promising early data we have seen on convalescent plasma. Data from studies conducted this year show that plasma from patients who have recovered from Covid-19 can help treat those suffering from the effects of this terrible virus.”
“At the same time, we will continue to work with researchers to pursue randomized clinical trials to study the safety and effectiveness of convalescent plasma in the treatment of patients infected with the novel coronavirus.”he added.
WHO urges caution
For its part, the World Health Organization is more measured on blood plasma treatment. “A number of clinical trials are underway around the world looking at convalescent plasma versus standard carerecalled at a press conference Soumya Swaminathan, executive director of the WHO. Only a few of them actually published intermediate results (…) and so far the quality of the evidence is still very low.”
.