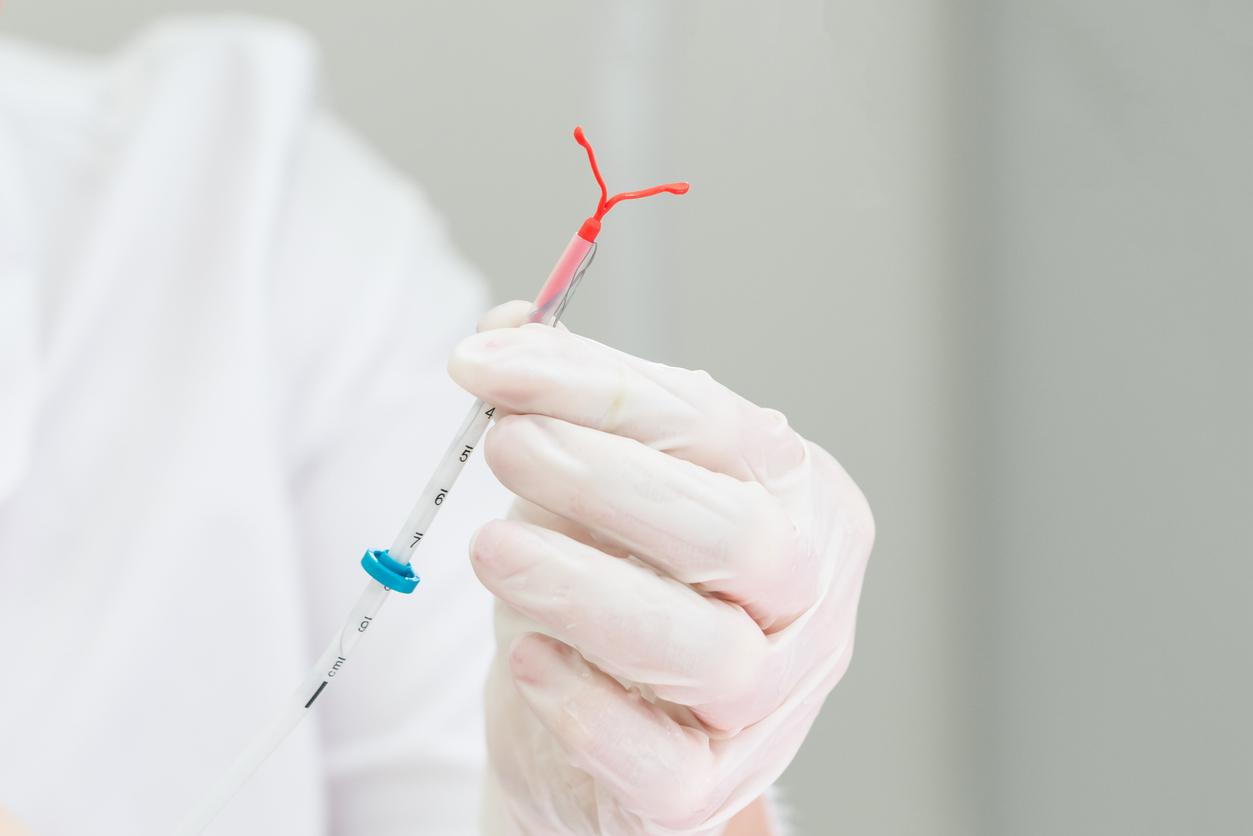
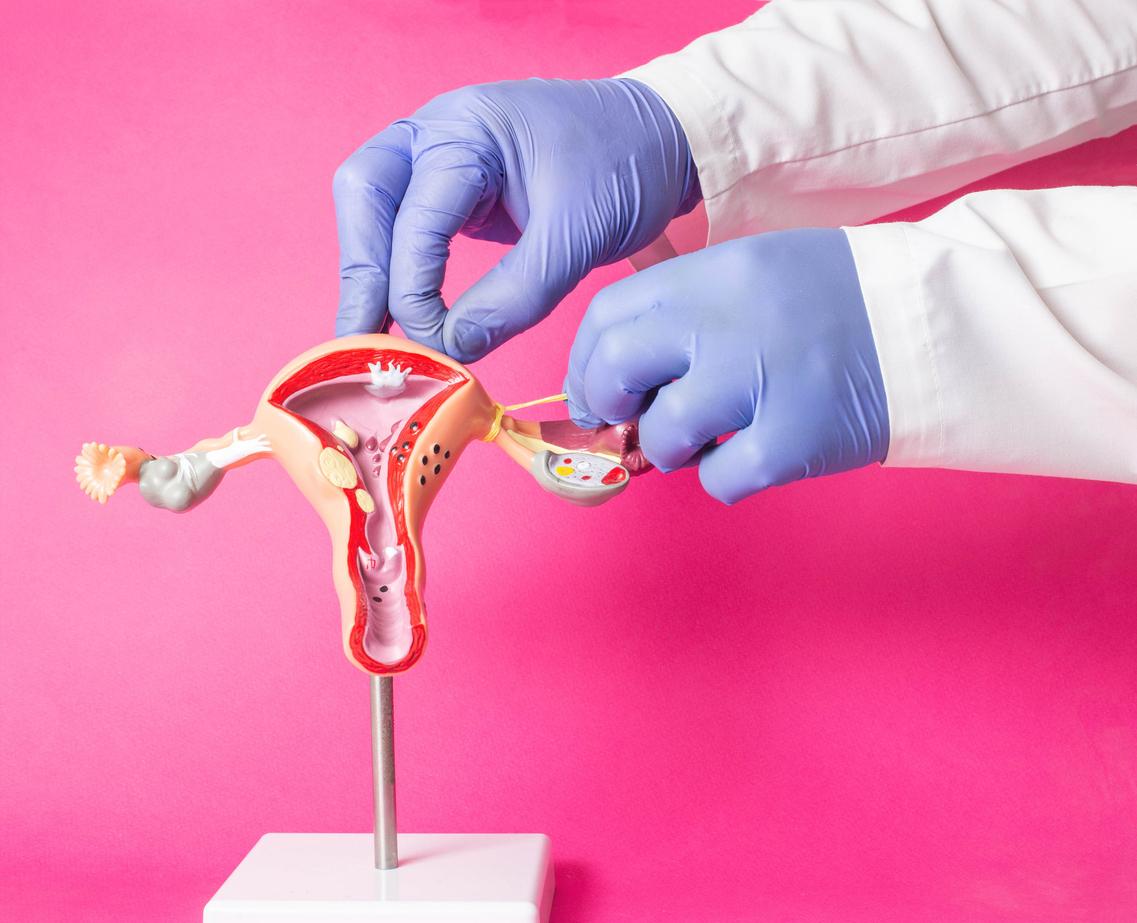
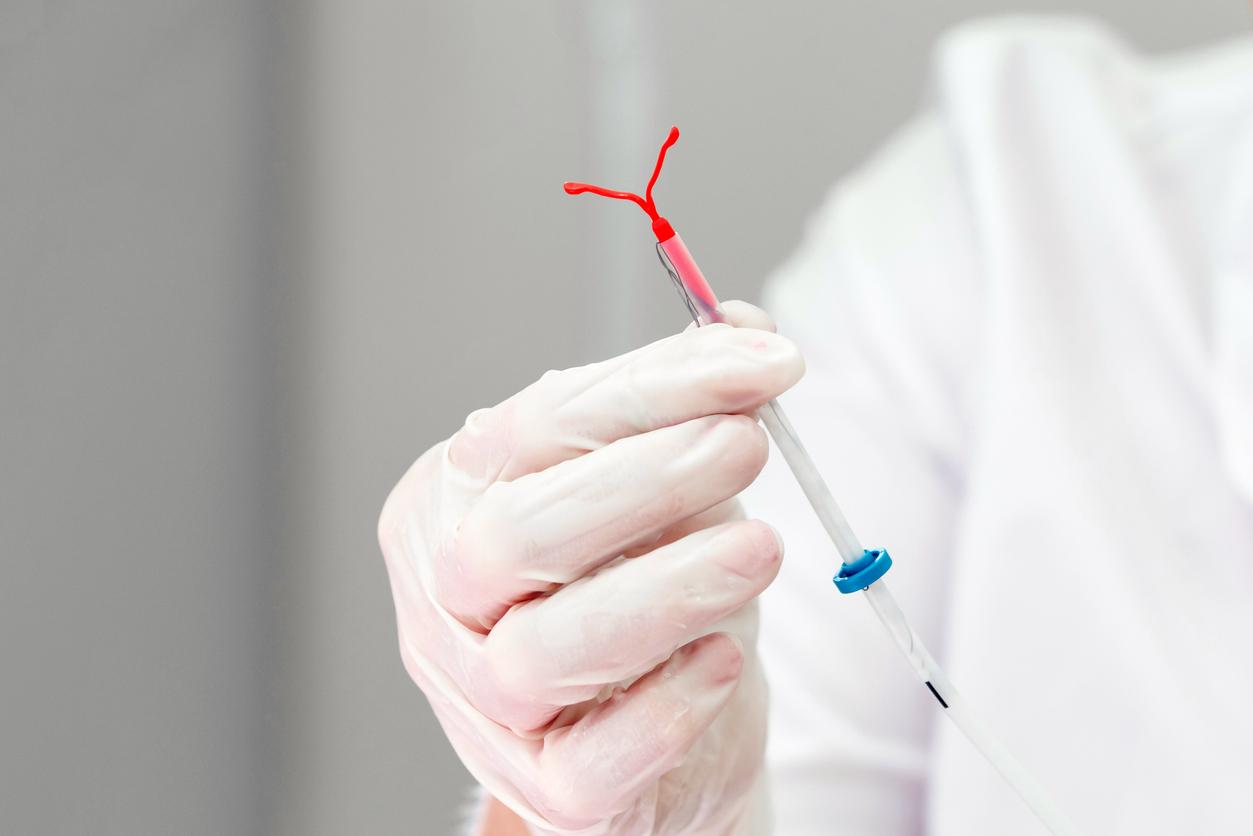
In a press release dated November 28, the Medicines Agency announced that it was suspending the marketing of three IUDs. In question: the risk of rupture during their removal and spontaneous expulsion.

The Ancora and Novaplus IUDs, marketed by Eurogin, and the Sethygyn IUD, from the manufacturer Euromedial, will no longer be marketed in France.
Thursday, November 28, the National Agency for Medicines and Health Products (ANSM) announced in a statement that these three intrauterine devices (IUDs) should therefore no longer be distributed and inserted. They are also the subject of a recall in the distribution circuit.
In question, specifies the ANSM: a level of information “insufficient as to the action to be taken in the event of partial or total expulsion of the IUD, when it is essential to consult your health professional in this situation”, but also a risk of rupture of the IUD during its removal.
Thus, it reminds the “companies which manufacture these IUDs, put them on the market or distribute them in France (Eurogine and Euromedial)” that they have “the obligation to withdraw all the IUDs concerned by this decision from all places where they are available.”
No removal of the IUD if it has been inserted for less than 3 years
Should the approximately 200,000 women wearing the Ancora, Novaplus and Sethygyn IUDs have their IUDs removed as a preventive measure?
No, recommends the ANSM, “when they have been in place for less than three years”. Beyond this period, however, the risk of deportation seems greater. It is therefore appropriate for the women concerned to contact their doctor.
The health agency also invites carriers of these three IUDs to “stay attentive to signs that may suggest expulsion”. Among these, “an absent or longer than expected IUD traction thread”, “abdominal pain”, “bleeding between periods or after intercourse” or even “pain during intercourse”.
However, some spontaneous IUD expulsions are asymptomatic
Also, if in doubt, the ANSM invites women to consult “as soon as possible the health professional who usually provides [leur] gynecological follow-up” and to use another method of contraception until the consultation.
It also recalls that an “expulsion of the IUD can call into question the effectiveness of contraception and expose to a risk of unwanted pregnancy” and that emergency contraception should therefore be considered for women who have had intercourse in the last five days after suspicion of deportation.

.