Six lots of the Diamilla contraceptive pill, produced by the Arrow laboratory, are being recalled. Stability studies have yielded non-compliant results.

Oral contraceptive users will need to check their medicine cabinet. Several lots of the Diamilla 75 microgram pill are being recalled by its manufacturer, Arrow Generics. The announcement comes from the National Medicines Safety Agency (ANSM). 6 lots are affected in total. This reminder is a precautionary measure. It “follows non-compliant results observed during stability studies”, specifies the ANSM.
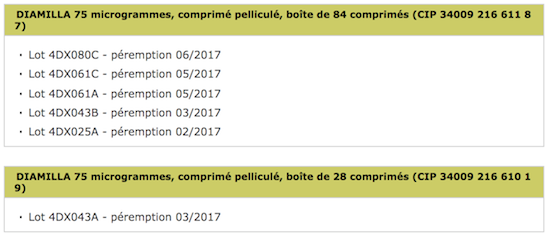
Source : ANSM
The pill in question is a generic that relies on progestin alone. The anomalies noted are, for the moment, of no consequence. No adverse effects related to the results were reported.
What is a stability test?
The stability tests mentioned by the ANSM are among the elements necessary for the marketing of a drug. They fulfill a main objective: to know how the final product, or its active principle, evolves according to various parameters. Ambient temperature, humidity, exposure to light are all factors that come into play. These studies must therefore take into account the area where marketing is planned.
These results are important: they determine the shelf life of a drug – indicated on the “expiration” date on the packaging. They will also influence the storage conditions recommended on the package leaflet. Failure to follow these rules can affect the effectiveness of the drug.
Strict rules
The World Health Organization (WHO) has set several rules regarding the conduct of these studies. A first must be carried out prior to placing on the market. “Samples are taken from two different production batches when the active ingredients are relatively stable,” says the UN health agency.
But the studies must also be repeated at regular intervals: for drugs considered to be stable, every two years; every three to five years for principles with established stability. If the production conditions or the composition change, the counters are reset to zero.

.