To cope with the supply difficulties which have weighed heavily on the pertussis vaccine for several months, the Ministry of Health has readjusted its vaccination strategy.
Vaccines against whooping cough are scarce. Significant supply tensions are to be expected during the year, and for a sustainable period, alert the National Medicines Safety Agency. The manufacturing laboratories, Glaxosmithkline and Sanofi Pasteur, even mention occasional shortages of stock.
Diphtheria-tetanus-pertussis-polyomyelitis
Four polyvalent vaccines (which contain several strains) are thus concerned: the two vaccines indicated in the joint prevention of diphtheria, tetanus, pertussis and polio (Infantrixtetra and Tetravac-acellulaire) and the vaccines indicated in joint prevention of diphtheria, tetanus, pertussis, polio and invasive Haemophilus influenzae type b infections (Infanrixquinta and Pentavac).
To cope with a possible shortage, the General Directorate of Health has decided to temporarily modify his vaccination strategy. To do this, it relied on the recommendations of the High Council of Public Health (HCSP).
Infants first
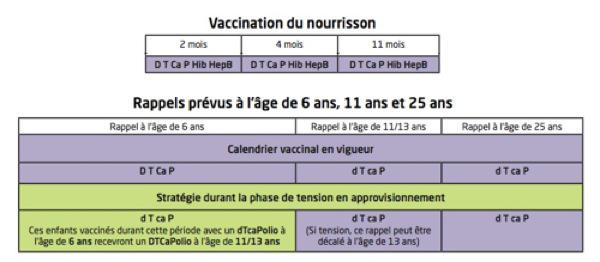
The latter believes that immunization of infants remains a top priority. Thus, for the very young, the vaccination schedule is maintained (2 months, 4 months and 11 months) but it will have to be based on the hexavalent infanrix Hexa® vaccine. Under no circumstances should vaccines containing reduced doses of antigens be used for the primary vaccination of infants or for the 11-month booster. The vaccine booster at the age of 11-13 years should be done with a tetravalent vaccine.
The cocooning strategy, which consists of vaccinating adults in contact with infants to protect the latter, is maintained. The tetravalent vaccine at a reduced dose of diphtheria toxoid and pertussis antigens can be given in children from 3 years of age.
Preserve stocks of pentavalent vaccines
The use of the hexavalent vaccine (Infanrix Hexa®) should be favored to preserve the stock of pentavalent vaccines for specific situations. In fact, newborns of mothers carrying the HBs antigen and those born in Guyana and Mayotte, who fall under a specific vaccination schedule, require the use of pentavalent vaccines for certain injections (Infanrixquinta® and Pentavac® ).
For newborns born to mothers who are carriers of the HBs antigen, the hepatitis B booster at the age of 6 months can be postponed to 11 months by using the hexavalent vaccine (Infanrix Hexa®).
The available doses of pentavalent vaccines (Infanrixquinta® and Pentavac®) will only be accessible in maternal and child health centers and vaccination centers.
.