For the first time, the European Medicines Agency has given the green light to the marketing of a cream against vitiligo.

- The American pharmaceutical company Incyte has obtained marketing authorization for its Opzelura cream from the European Medicines Agency.
- This treatment, recommended for adults and adolescents from 12 years old, must be applied twice a day, for 6 to 24 months, for complete repigmentation of the skin.
- The drug, which has been shown to be effective in 31% of patients, should be available in pharmacies in the coming months.
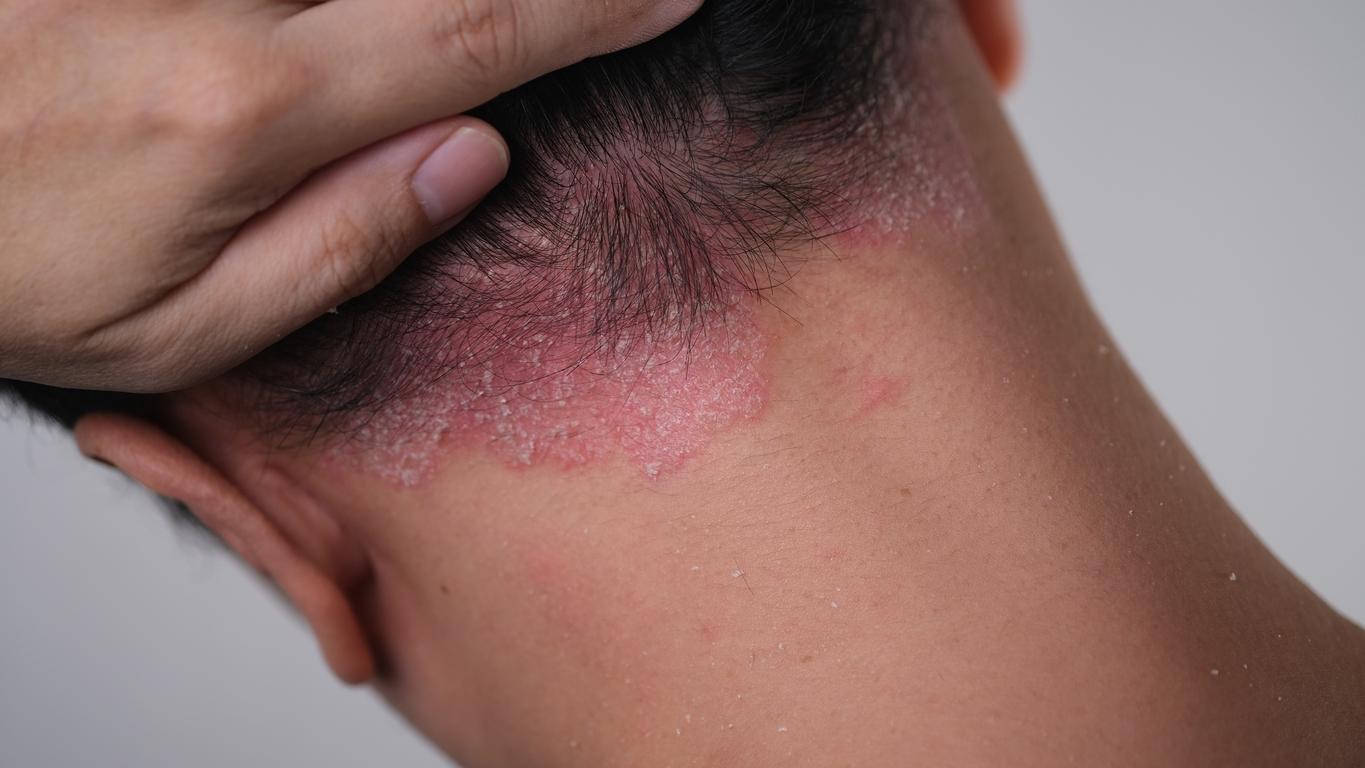
Long misunderstood, vitiligo affects up to 2% of the world’s population. In France, it concerns 600,000 to one million people. As a reminder, this is a disease considered “autoimmune” during which white spots appear on the skin. This pathology “is linked to the loss of cells present in the epidermis (the superficial layer of the skin) called melanocytes which have the role of producing melanin: the pigment of the skin”, noted Bordeaux University Hospital.
Opzelura cream against vitiligo would be effective and “very well tolerated”
On April 20, the European Medicines Agency gave the green light to the marketing of Opzelura (or ruxolitinib) cream against vitiligo. This treatment, developed by the American pharmaceutical company Incyte, makes it possible to re-pigment the skin in adults and adolescents over the age of 12. The agency approved it after viewing the results of clinical trials evaluating the cream. A total of 600 people with vitiligo took part in the research, which concluded that the product was effective in 31% of patients.
“Two major international studies have confirmed its effectiveness, particularly on the face. Thus, with an application twice a day, for six to twenty-four months, the re-pigmentation is complete. We observed a stability of positive results but, if the vitiligo persists, it is possible to redo the treatment”, explained, to the newspaper 20 minutes, Thierry Passeron, head of the dermatology department at the University Hospital of Nice and member of an international group of experts who developed the product. He specified that the cream was “very well tolerated” And “did not require a blood test”. However, “some areas are difficult to re-pigment, such as the feet or the hands”.
Vitiligo: a treatment that “should be in pharmacies in the coming months”
“It’s the end of the therapeutic desert”, declared, at Sunday newspaper, Julien Seneschal, head of the inflammatory and autoimmune dermatology unit at Bordeaux University Hospital, who took part in international studies. He felt that this first drug opened “the way to other innovative treatments”. According to the president of the French Vitiligo Association, Martine Carré, Opzelura cream “should be in pharmacies in the coming months” and the terms of reimbursement will soon be specified.