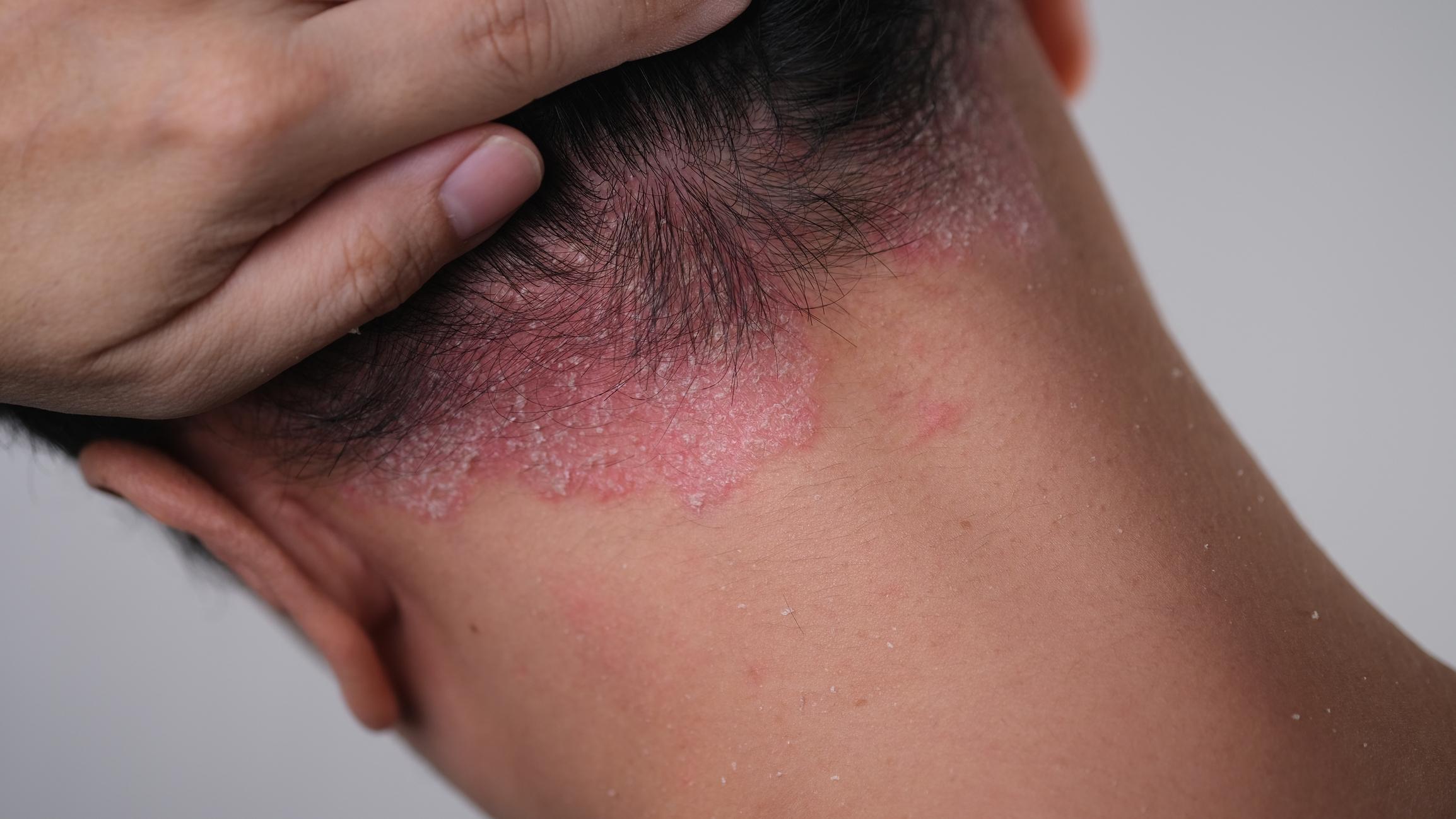
Efaluzimab (Raptiva) has been marketed in France since 2005. It is indicated for the treatment of adult patients with moderate to severe chronic (long-term) plaque psoriasis. Dermatologists use it in patients who have not responded to other systemic treatments (which spread throughout the body) for psoriasis or who are intolerant to these types of treatments, such as ciclosporin, methotrexate and PUVAtherapy (psoralen and ultraviolet A).
The Medicines Evaluation Agency (EMEA) has revised its marketing authorization at the request of the European Commission, which was alerted by the occurrence of 3 cases of progressive multifocal leukoencephalopathy (PML), in patients with taken the Raptiva for over 3 years. Progressive multifocal leukoencephalopathy (PML) is a serious, progressive brain infection that can lead to death or severe disability. Following these cases, Emea concluded that the risk-benefit ratio of efaluzmab had become unfavourable.
To date, the French Agency for the Safety of Health Products (Affsaps) estimates that 560 patients are being treated. It recommends that they do not suddenly interrupt this treatment but consult their prescribing doctor quickly in order to set up a replacement treatment.
To learn more about the disease: Treat psoriasis well