Urinary biomarkers can shed light on the amount of cancerous cells in a prostate, indicating with more certainty which men need treatment.
- About half of men diagnosed with prostate cancer have an intermediate risk.
- This urine test assesses risk without the need for an invasive biopsy.
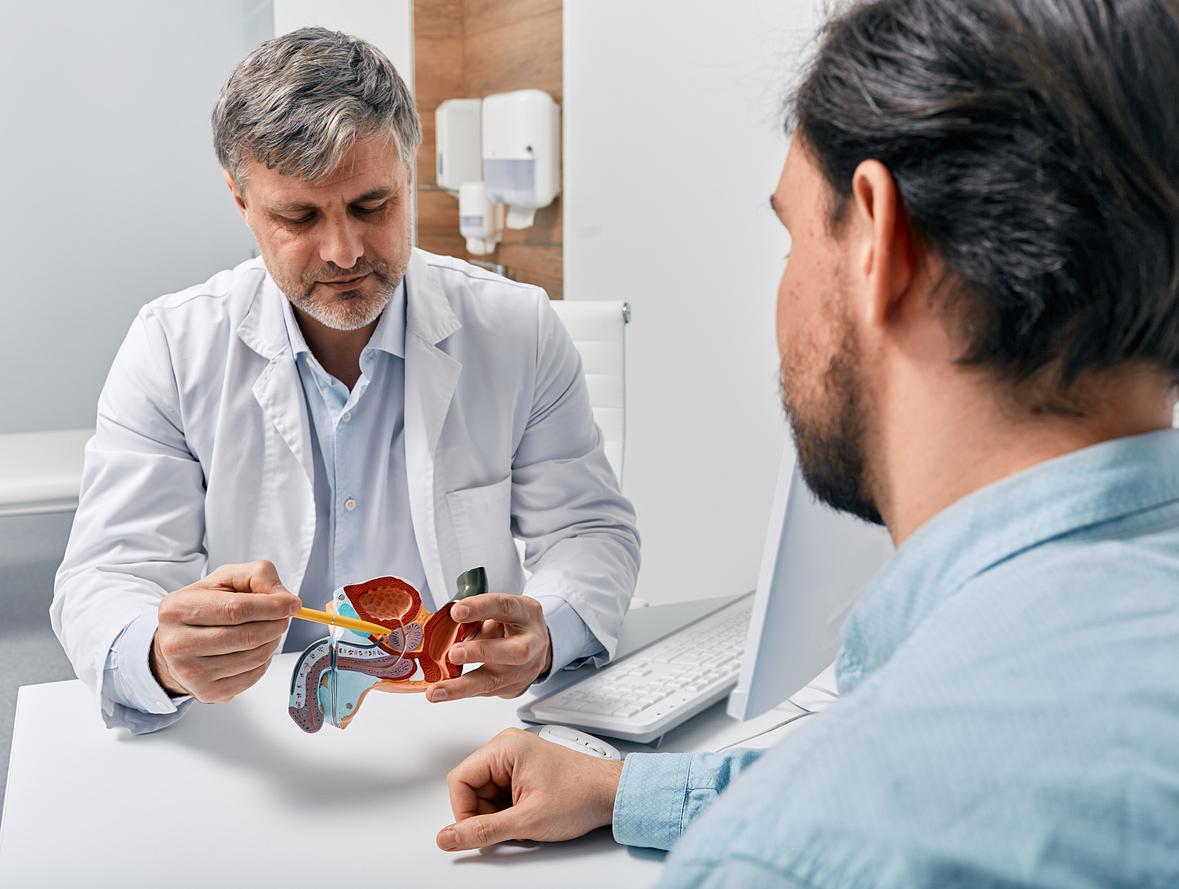
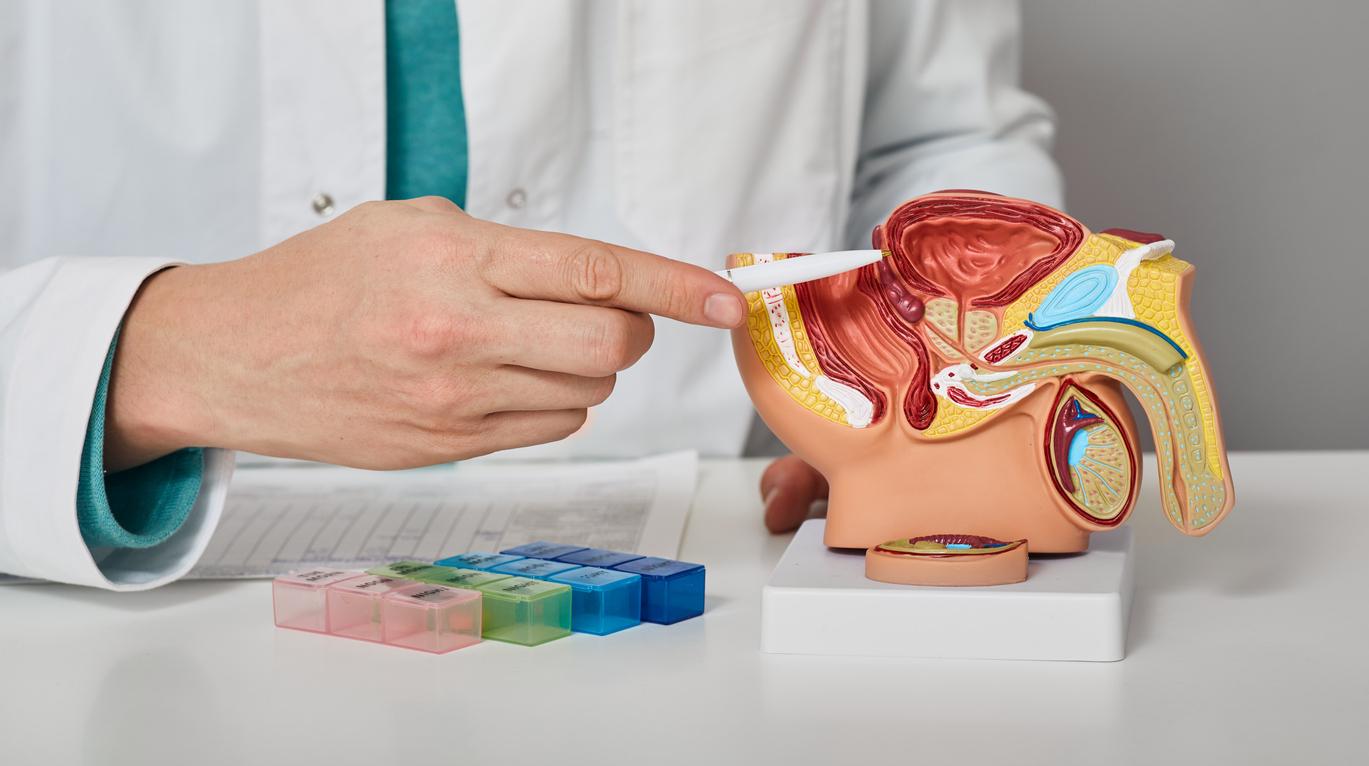
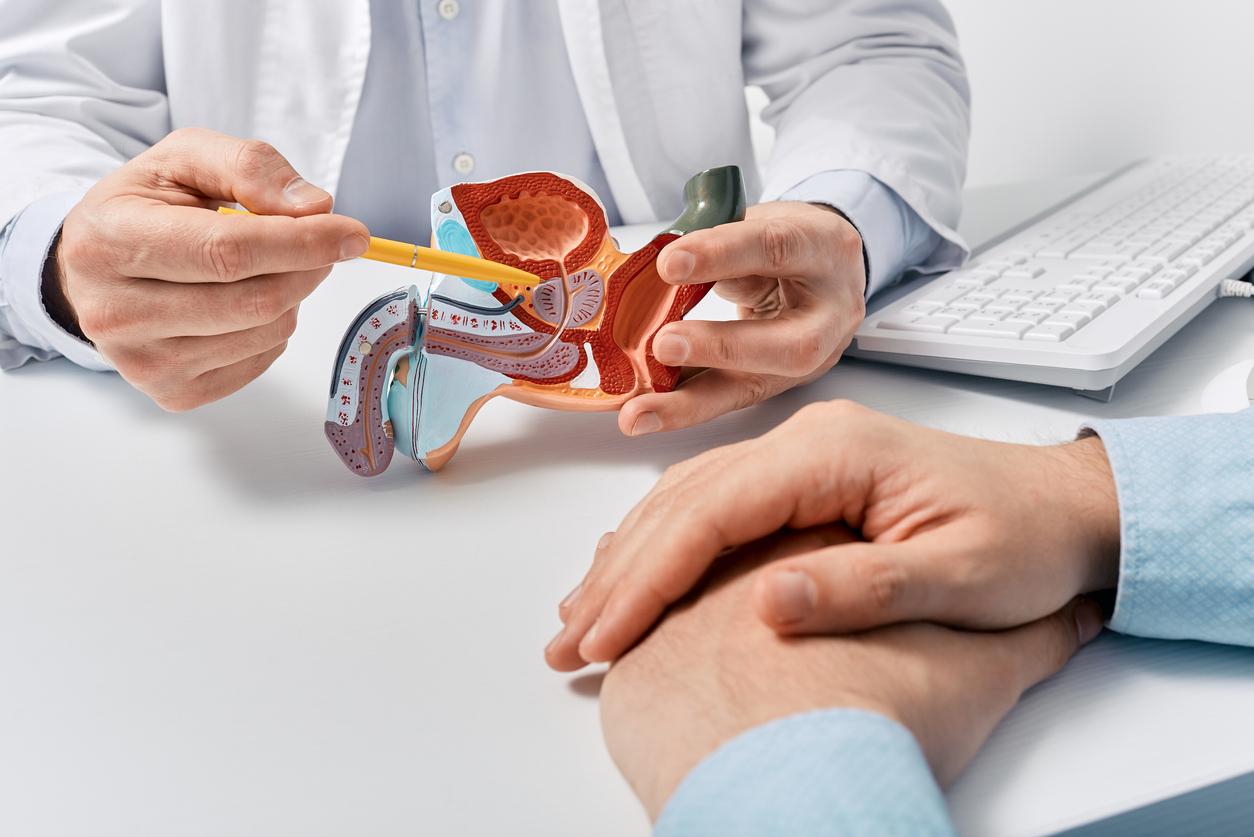
Movember is an annual event that raises awareness about male diseases such as prostate cancer, the most common in men. It is rather rare before the age of 50: the majority of cases are detected around the age of 70. Two examinations make it possible to detect it: the digital rectal examination and the dosage of the PSA, the specific antigen of the prostate. But in patients who have been diagnosed with cancer, a simple urine test would identify those who are at risk and who need active surveillance. This is revealed by a study conducted by British researchers from the University of East Anglia, the results of which were published in the journal Life.
Diagnose men at “intermediate” risk
The Prostate Urine Risk (PUR) test is already able to identify men with high and low risk cancers. Researchers have shown in this new study that with a few tweaks, it is now possible to detect men with “intermediate” risk disease for whom treatment options are less obvious and for whom doctors find it difficult to predict which tumors will progress to a more aggressive form. The risk refers to the aggressiveness of the cancer and its potential to spread to other organs, which would eventually kill the patient, say the researchers.
The study authors estimate that about half of men diagnosed with prostate cancer fall into this middle group. “Disease progression in men at intermediate risk is known to be associated with the presence of increasing amounts of Gleason type 4 cancer in their prostates.adds Dr. Jeremy Clark, lead author of the study. Our study shows that the PUR test can assess the amount of Gleason 4 pattern without the need for a biopsy. So not only can PUR measure the presence of aggressive cancer, but it can also measure increasing amounts of aggressive cancer in the prostate..”
Avoid biopsy
The researchers plan to test their new test on a larger cohort of patients. “We look forward to seeing further validation of this research in a larger study cohort.says Sarah Hsiao, director of biomedical research and impact at Movember, commenting on the article. If successful, this non-invasive PUR test may be able to support the decision-making process without the need for an invasive prostate biopsy which is associated with discomfort and risk of infection..”

.