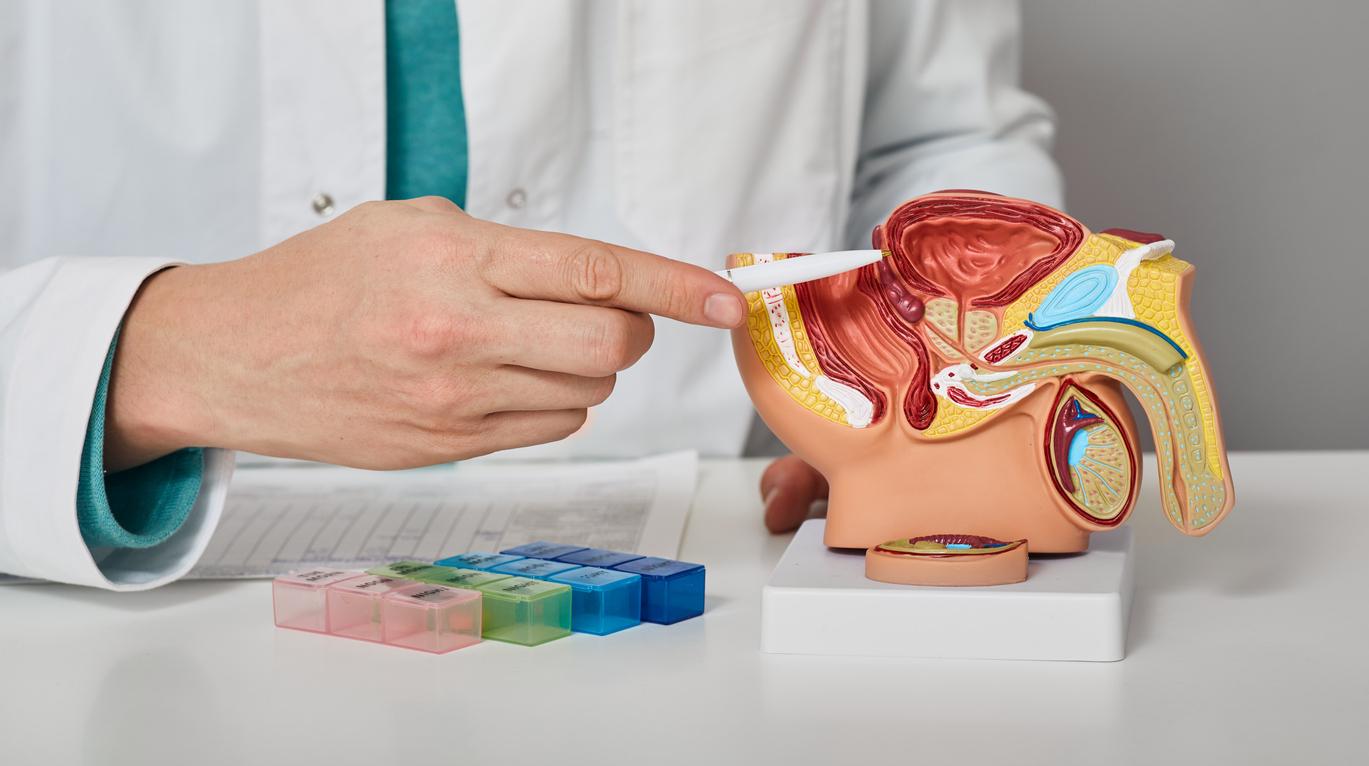
Combining two hormone therapies and chemotherapy halves the risk of tumor progression in prostate cancer.
- With some 50,000 new cases diagnosed each year, prostate cancer is the leading male cancer.
- It is also ranked 3rd in terms of deaths in humans: in 2018, it caused 8,100 deaths in France.
The European PEACE 1 study could be a game-changer and change the standard treatment for metastatic prostate cancer. The first results of this phase III clinical trial promoted by Unicancer, presented at the ASCO 2021 congress by Pr Karim Fizazi, oncologist at Gustave-Roussy, show that combining abiraterone from the outset with conventional hormone therapy and chemotherapy halves the risk of tumor progression and increases disease progression-free survival by two and a half years.
10% of prostate cancers are metastatic
Approximately 10% of prostate cancers diagnosed each year turn out to be metastatic from the outset: for these 5,000 patients, the prognosis for long-term survival is not high. The management of these tumours, which also turn out to be more aggressive, has long been based solely on the inhibition of testosterone production, which feeds these hormone-sensitive tumours, recounts Professor Karim Fizazi. “Since the removal of the testicles, historically practiced in the 1940s, much has evolved to suppress testosterone production. Classic hormone therapy drugs have thus become the standard for the management of these hormone-sensitive metastatic cancers” .
For several decades, starting in the 1980s, they were the only treatment option but generally only gained one year of survival without disease progression. A major revolution began in 2015 with new options, which broadened the therapeutic arsenal and made it possible to intensify treatments. Several studies and meta-analyses have successively demonstrated that immediately combining taxane-based chemotherapy (docetaxel) with classic androgen suppression increases the median survival time without progression of the disease to approximately 2 years. Recent data on new generation hormone therapies (abiraterone, apalumide or enzalutamide) have also shown that they improve the results of traditional hormone therapy alone. Both options have become the recommended standard of care, with oncologists choosing one or the other. “In the absence of data evaluating the benefit of adding the different treatments from the outset, the question of combining them, when and for how long remained unresolved”, explains Professor Fizazi.
Three drugs instead of two
The European PEACE 1 study aimed to fill this gap, by comparing the benefit of a combination of three drugs instead of just two, on two main criteria: disease progression-free survival and overall survival. The preliminary results on the first evaluation criterion already plead clearly in favor of the simultaneous combination of the three therapies from the outset: abiraterone + conventional hormone therapy + chemotherapy.
The clinical trial was conducted between 2013 and 2018 on a cohort of 1,173 patients, with a median age of 67, recruited from 80 hospitals in seven European countries (France, Spain, Italy, Switzerland, Ireland, Belgium and Romania) . All had recently diagnosed metastatic prostate cancer, but could, on inclusion, have already started treatment with conventional hormone therapy for less than three months. Randomly divided into two groups, some received a treatment combining the three therapies: classic hormone therapy coupled with 6 cycles of docetaxel (75 mg/m2), at a rate of one every three weeks and 1,000 mg of abiraterone/day . The control group was treated with a combination of two therapies: classic hormone therapy + chemotherapy.
Follow-up at 42 months “shows a very clear improvement in radiological progression-free survival in the group treated additionally with abiraterone, regardless of the number of metastases at diagnosis”, notes Professor Fizazi. There is indeed a difference of two and a half years between the two groups of patients. “Gaining an additional two and a half years, without symptoms or elevation of disease biomarkers and thus allowing an overall progression-free survival time of 4 and a half years, is unheard of! Additional good news: at six months, the combination of the three treatments did not induce any significant additional toxicity. We did not observe any side effects other than those classically attributable to abiraterone, which were mostly benign”, rejoices Karim Fizazi.

.