Suffering from a rare and incurable pediatric disease, the little patient had received gene therapy treatment several months ago as part of a clinical trial.

- A girl participating in a clinical trial evaluating a gene therapy treatment for Sanfilippo A disease has died in the United States.
The French biotech Lysogene announced on Thursday October 15 in a press release that a girl participating in a clinical study evaluating a gene therapy treatment died in the United States.
The five-year-old patient suffered from a rare and incurable pediatric disease. It is in this context that she participated in this international clinical study in phase 2/3, on a phase of test procedure in three phases. At this time, the immediate cause of his death remains unknown. “At this stage, there is no evidence that this event is related to the administration of the product”says Lysogene in its press release.
Questioned by AFP, the company specifies that the girl was not hospitalized at the time of her death. She had already received her treatment in one of the four American centers participating in the clinical trial and had returned to her home.
A suspension of the clinical trial
Consisting of introducing genetic material to “correct” the cells of a patient, gene therapy is now seen as a real hope in the treatment of rare diseases, cancers or neurodegenerative diseases.
The LYS-SAF302 clinical trial in which the little girl took part aimed to develop a gene therapy for Sanfilippo disease type A, a pediatric neurodegenerative disease of genetic origin, which is characterized by the abnormal accumulation of compounds called glycoaminoglycans ( or mucopolysaccharides) in body cells. This accumulation occurs mainly in the brain, but also in the lungs and bones and mainly leads to behavioral disorders, respiratory damage and abnormal bone development. Sanfilippo type A disease causes rapid and severe intellectual decline.
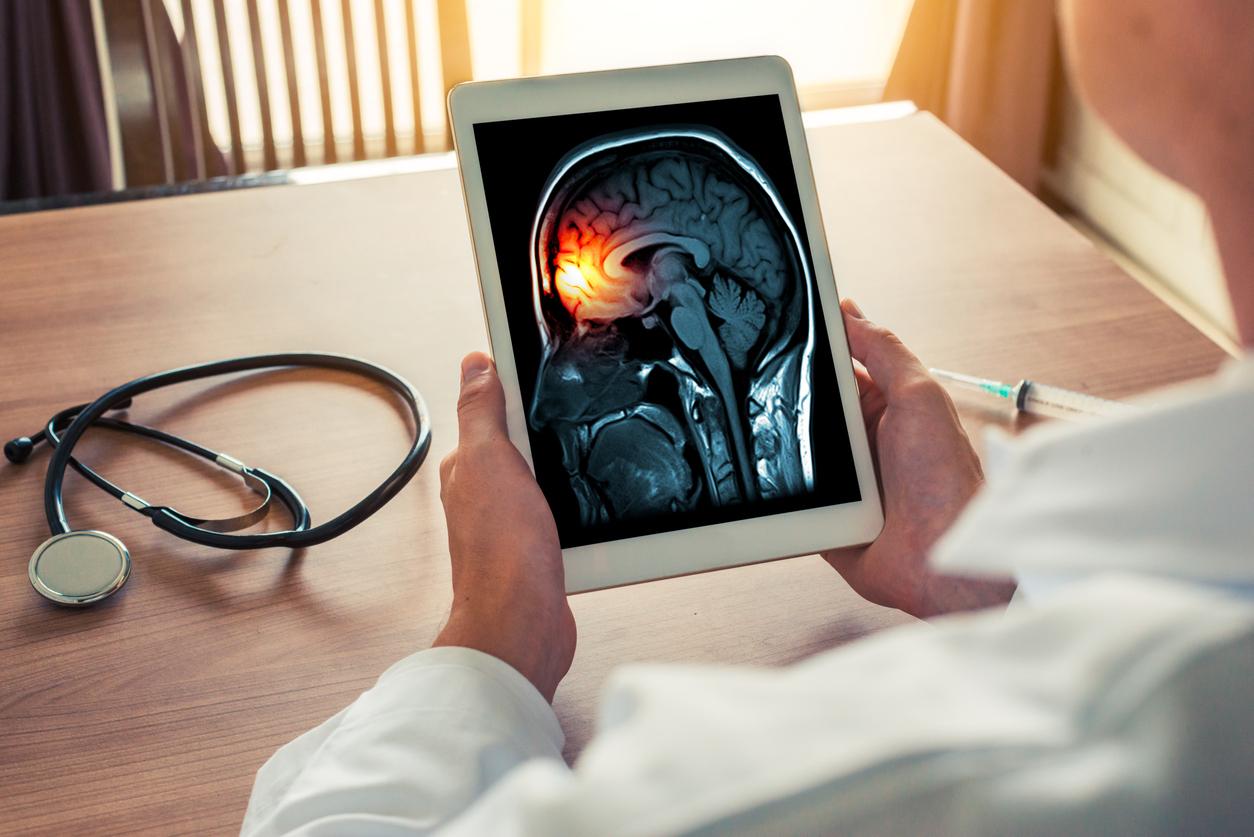
Already last June, the Food and Drug Administration (FDA) had requested the suspension of the clinical trial after “localized signals at intracerebral injection points” were observed on MRI images. The FDA had then estimated that there was a “potential link to product administration” even if “no clinical symptoms can be directly attributed to these MRI observations”.
18 patients still followed
To date, 19 of the 20 patients participating in the study have received the product. The 18 patients continue to be followed and are “enough” according to Lysogene to continue the study and develop a treatment for Sanfilippo type A disease. The company said to itself “deeply saddened by the death of this child” and ensures to be compiling “additional information” to determine the cause of his death.
.