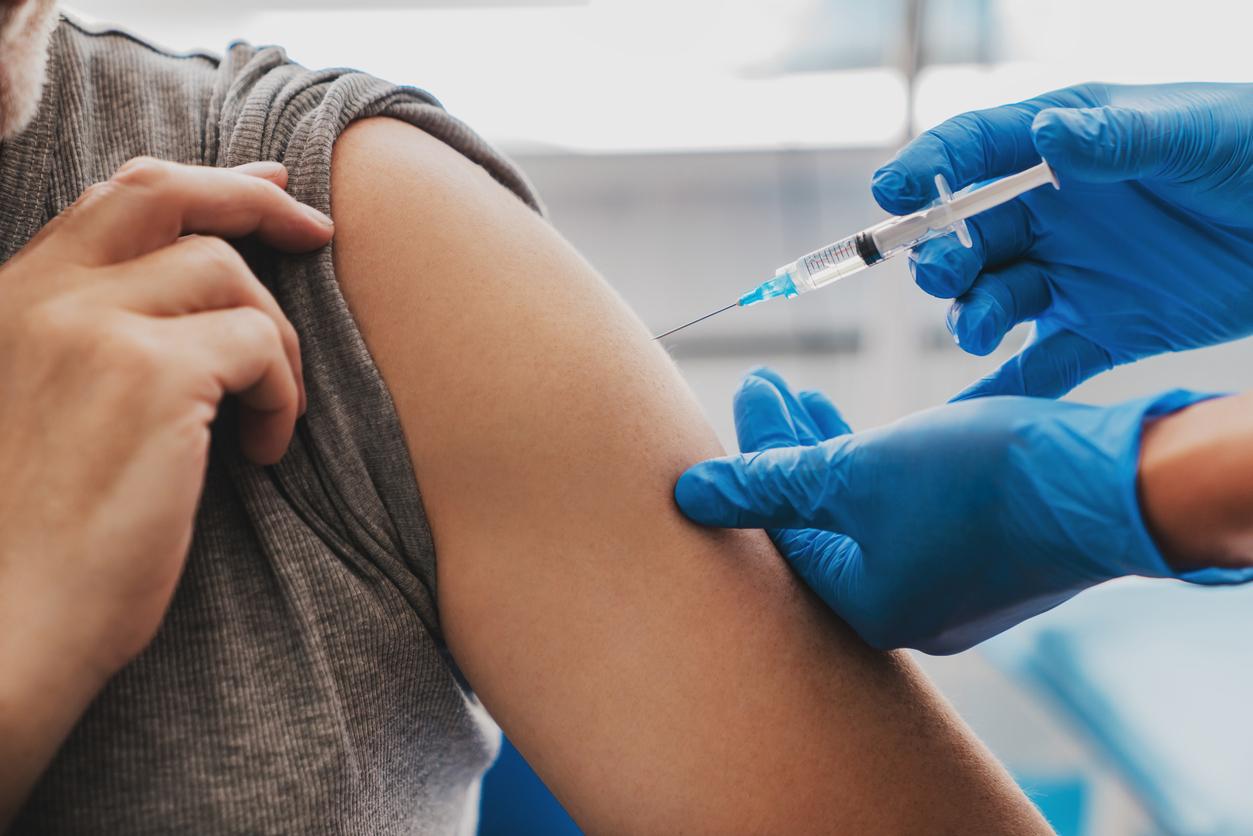
US health authorities have urgently authorized the single-dose vaccine from Johnson & Johnson. A hope for France which could have this product at the end of March-beginning of April while health professionals are getting impatient with the supply difficulties concerning the vaccines already available.

- Johnson & Johnson’s vaccine urgently authorized in the United States
- In France, doctors are waiting for an acceleration of the authorizations of new products
Looking for doses of vaccine, desperately… “It is very important that Europe speeds up its authorization procedures for all these new vaccines, including those of the Russian vaccine, the more new vaccines we have, the more we will manage to surpass the constraint of production chains which have limited capacities”, declared on February 28 on France Info Professor Djilalli Annane, head of the intensive care unit at the Raymond Poincaré hospital in Garches.
A wish that comes at a time when the vaccination campaign in France is coming up against the difficulty for many centers of finding the doses to meet the demands of the categories of the population who now have access to the vaccine. And the same day that Dr. Jean-Paul Ortiz, president of the CSMF (Confederation of French Medical Unions) deplored on the same media the delay, due to the impossibility of obtaining the necessary doses of the AstraZeneca product, in vaccination by general practitioners aged 50-64 with comorbidities in open theory since February 24.
“27 patients waiting, and still nothing…”
“It’s starting slowly, Jean-Paul Ortiz told us on March 1, it depends on the location, but I was in contact this morning with a GP from Poitiers who has 27 patients waiting and who, to date, has still not received nothing”.
Glimmer of hope in this landscape, the green light granted on February 27 by the American Medicines Agency (FDA) to the Johnson & Johnson vaccine, for which authorization was given urgently following the publication of data from clinical trials on more of 43,000 people. A decision which should, according to the JDD, make it possible to accelerate the American campaign while nearly 3 million doses of this product should be distributed this week across the Atlantic.
And according to the Bloomberg agency, also quoted by the JDD, Europe could in turn quickly benefit from this new vaccine since the European Medicines Agency should issue an opinion on the application for authorization of this vaccine as of March 11. filed with her on February 16.
A new vaccine available in France at the end of March-beginning of April
For France, the first doses could be available at the end of March or the beginning of April, according to the declarations of Agnès Panier Runacher, Minister Delegate for Industry, Sunday February 28 on France 3. The effect of the arrival of this vaccine on the vaccination campaign in France should be important since it is effective with a single dose and can be stored in a simple refrigerator, which greatly simplifies the logistics necessary for supplying health professionals.
This vaccine, which has been baptized Janssen Covid-19 and whose announced efficiency would be 85%, is a product which uses a low-virulence virus carrying genetic instructions which generate the production by cells of a SARS-CoV-protein. 2 to help the immune system recognize this virus responsible for Covid-19.
.