The High Authority for Health (HAS) gave the green light on Saturday to the deployment of antigenic tests for “contact persons”. Faster than RT-PCR virological tests, they have so far been reserved for people symptomatic of Covid-19.
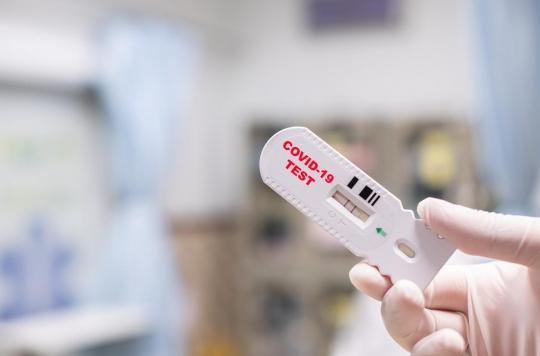
- Until now reserved for symptomatic and asymptomatic people in the event of unavailability of RT-PCR tests, the antigen test will now be accessible to contact cases, announced the HAS.
Developments in the diagnostic and screening strategy for Covid-19. Hitherto reserved for “asymptomatic people, excluding contact people or people detected within a cluster”as well as to “symptomatic people” in the event that an RT-PCR test could not be obtained before 48 hours and within 4 days after the first symptoms, antigenic tests will soon be generalized to contact cases.
In a statement dated Saturday, November 28, the High Authority for Health (HAS) said to itself “in favor of extending the indications for antigenic tests, in order to use them not only in people with symptoms, but also in contact people detected in isolation or within clusters”.
If we now have to wait for a decree published in the Official Journal for these terms to become effective, this green light opens the way to widespread use and reimbursement of these antigenic tests.
An efficient and rapid diagnosis
To justify its decision, the HAS explains that it relied on “new scientific publications (…) reassuring as to the ability of antigenic tests to effectively diagnose contact persons” even if she admits that their use “is however still not recommended for the screening of isolated asymptomatic people, for lack of data”. A position subject to review “function of the evolution of scientific knowledge”.
The period of use of antigen tests “is the same as that recommended for RT-PCR, namely: as soon as possible then at seven days for high-risk contact persons (in the same household as an infected patient); at seven days after exposure for other contact persons (low risk)”specifies the High Authority for Health.
Faster but less sensitive to the virus EasyCov saliva tests
HAS also announces that it is in favor of the deployment and use of tests EasyCov saliva “in symptomatic patients for whom nasopharyngeal sampling is impossible or difficult to perform“. Quicker to perform than RT-PCR virological tests, these tests also make it possible to obtain a result in about forty minutes, compared to several hours for nasopharyngeal tests. They nevertheless prove to be less efficient because they are less sensitive to the virus than RT-PCRs.
Too, “the absence of robust clinical data on the diagnostic performance of EasyCov in asymptomatic people does not allow it to be recommended, at this stage, in this situation”.
.