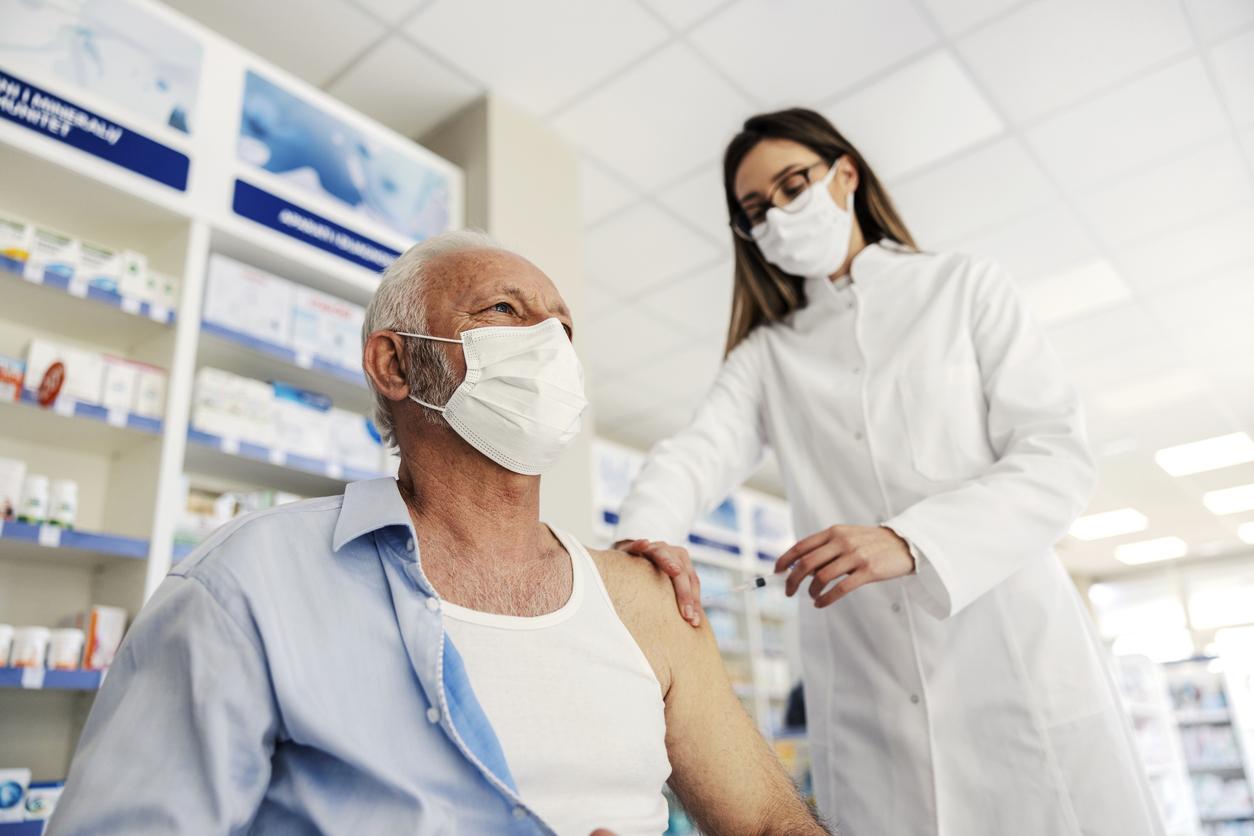
At first greeted with suspicion, the effectiveness of the Sputnik vaccine has been demonstrated by independent scientists. German Chancellor Angela Merkel says she is “ready to support Russia” in her marketing authorization application to the European Medicines Agency.
Impressive results
The results of the Russian vaccine Sputnik V clinical trials were validated by independent experts and published in the prestigious scientific journal The Lancet on Tuesday, February 2. They assess the effectiveness of the Russian vaccine at 91.6% against symptomatic forms of Covid-19, thus corroborating Russia’s initial claims. This means that the independent review committee has removed any remaining doubts about this vaccine. Henceforth, it is therefore one of the most effective anti-covid vaccines at this stage, along with those from Pfizer / BioNTech and Moderna.
Another weapon against the pandemic
“The development of the Sputnik V vaccine has been criticized for its haste, the fact that it has skipped steps and a lack of transparency. But the results reported here are clear and the scientific principle of this vaccination has been demonstrated, ”said two British specialists, Professors Ian Jones and Polly Roy, in a commentary attached to The Lancet study. This “means that an additional vaccine can now join the fight to reduce the incidence of Covid-19”, insisted these researchers, who were not themselves involved in the study. Already used in several countries including Russia, Argentina and Algeria, Europe is awaiting marketing authorization from the European Medicines Agency (EMA).
How effective on asymptomatic patients?
Carried out on a cohort of 20,000 people, the clinical trials took place between September and November. The participants all received two doses of vaccine or placebo three weeks apart. In the days following the administration of the second dose, a PCR test was performed in people with symptoms of covid. As a result, only 16 volunteers out of 14,900 who received the two doses of the vaccine tested positive, ie 0.1%, against 62 of the 4,900 who had received the placebo (ie 1.3%). The authors of the study qualify, however, by explaining that these tests were carried out “only when the participants declared to be suffering from symptoms of Covid, the analysis of the effectiveness relates only to the symptomatic cases”. Further research therefore needs to be carried out to be able to assess the efficacy of the vaccine on asymptomatic cases and on disease transmission.