Invited this Sunday, March 14 on the set of BFM TV, the director general of health, Jérôme Salomon announced the imminent arrival of the Covid-19 self-tests. Already marketed in other European countries, these are antigenic tests which allow results in less than 20 minutes but whose effectiveness and traceability are yet to be defined …
Self-tests arrive in France
After Germany, the United Kingdom, Austria or even Portugal, France is preparing in turn to authorize the sale of self-tests to screen for Covid-19. Indeed, the Director General of Health, Jérôme Salomon, announced this Sunday, March 14, 2021 during a televised interview on BFMTV, that self-tests would be available this week in France. He thus specified that the latter will be available ” in pharmacies or supermarkets “.
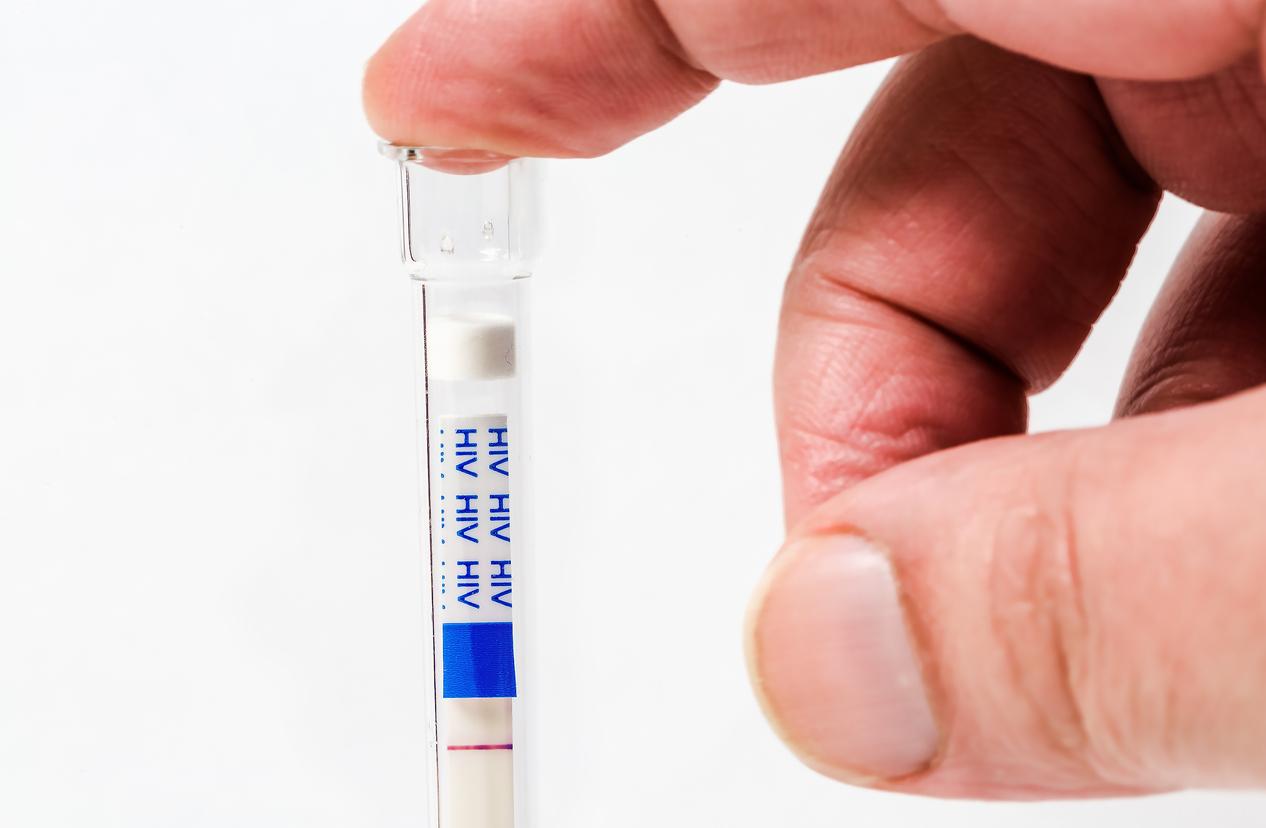
These self-tests are antigen tests. In other words, these are tests that are performed by nasopharyngeal swab but unlike PCR tests, self-tests do not need to be introduced so deep into the nose since it suffices to go to the surface and not deep in the nasopharynx. Once entered in the nose, the sample is to be placed in a tube. The results are displayed in the same way as a pregnancy test in less than 20 minutes.
Reliability and traceability of self-tests
Before being marketed, self-tests must be submitted to the opinion of the Haute Autorité de Santé. And for good reason, the effectiveness of the tests is still debated. According to Jérôme Salomon, “ the real issue for me is scientific evaluation. We cannot allow tests that would give false negatives, or false positives. It is therefore necessary to be certain that these tests are reliable and that the French can have a confirmation of the test. “.
The other issue relating to the marketing of self-tests concerns their traceability to health authorities and health insurance. In order to organize the tracing of contact cases and control the isolation of positive cases, self-tests must be taken into account in the strategy of the contact tracing system. As mentioned by Jérôme Salomon “ the real question is how we trigger the ‘Test-Alert-Protect’ device to track the person “. This is why before the antigenic self-tests are marketed in supermarkets and pharmacies, the Haute Autorité de santé will have to set the approval conditions for Sars-CoV-2 screening kits to be used by oneself.