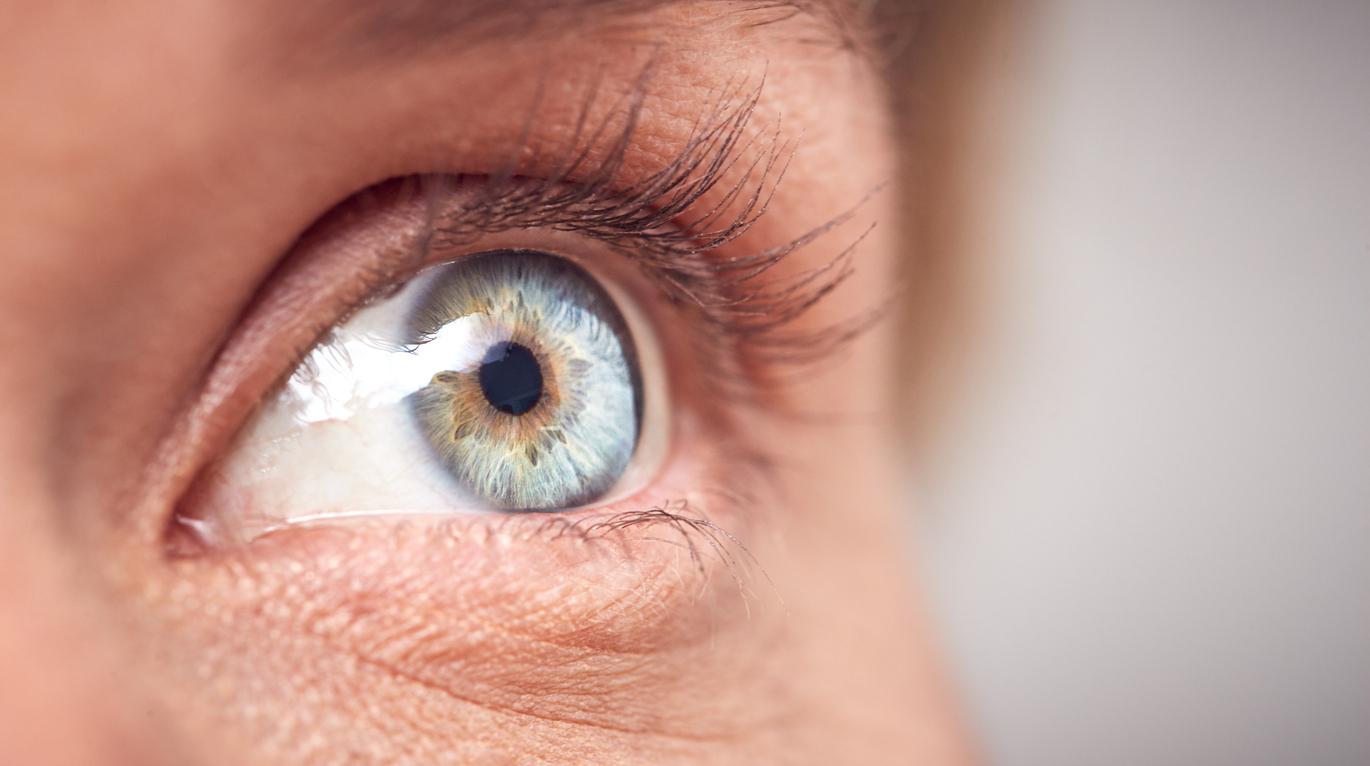
The injection of copies of a missing gene that causes partial or total vision loss at birth or in early childhood has improved the sight of the first three child-patients after nine months of follow-up.

- Injection of functional copies of the GUCY2D gene resulted in improvements in some aspects of vision, without serious side effects.
- Gene therapy consisted of injecting functional copies of the GUCY2D gene, implicated in many cases of childhood blindness.
- No side effects were reported.
- Future patients will receive larger doses of gene therapy.
Is childhood blindness on the way out? Currently, about 1.5 million children suffer from partial or total blindness from birth or in early childhood. Around 25 different genes are involved, with mutations causing problems with the retina and causing severe visual impairment. Among them is the GUCY2D gene, the absence of which is one of the main causes of childhood blindness. Thanks to gene therapy, American researchers from the Scheie Eye Institute in Perelman School of Medicine from the University of Pennsylvania succeeded in improving the vision of child patients by injecting them with functional copies of the GUCY2D gene.
Three patients, three improved views
A total of three patients received gene therapy. They all experienced improvement in some aspect of vision, with no serious side effects. Their cases were presented in a scientific publication published on April 6 in the journal iScience. “We saw long-lasting improvements in day and night vision, even with a relatively low dose of gene therapy”, specified Samuel Jacobson, professor of ophthalmology at the University of Pennsylvania. The dose of gene therapy used in these first three patients was the lowest of the doses the researchers plan to use in the study. They hope to see continued safety and greater efficacy in patients recruited later, who will receive higher doses.
In detail, the first patient experienced a substantial increase in light sensitivity in rod cells, which are more sensitive to light than cone cells and are primarily useful for vision in low light. It also showed improved pupil responses to light. The second patient showed a smaller but sustained increase in light sensitivity in rod cells, beginning about two months after gene therapy. Finally, the third child did not experience any improvement in rod cell sensitivity but showed much better visual acuity during the 9 months of follow-up. Researchers have linked it to better functioning of the patient’s cone cells, the predominant cells for daylight and color.
Reactivate cells and restore vision
“Normal” copies of the GUCY2D gene encode an enzyme in the key pathway that light-sensitive rod and cone cells in the retina use to convert light into electrochemical signals. The absence of this enzyme leads to a block in the recovery of this pathway, preventing the resetting necessary for subsequent signaling. As a result, the signal from rod and cone cells becomes very weak. As a result, it leads to severe vision loss. In many patients, many light-sensitive retinal cells may remain alive and intact despite their dysfunction. The addition of functional copies of GUCY2D via gene therapy makes it possible to reactivate these cells and restore some vision.
.