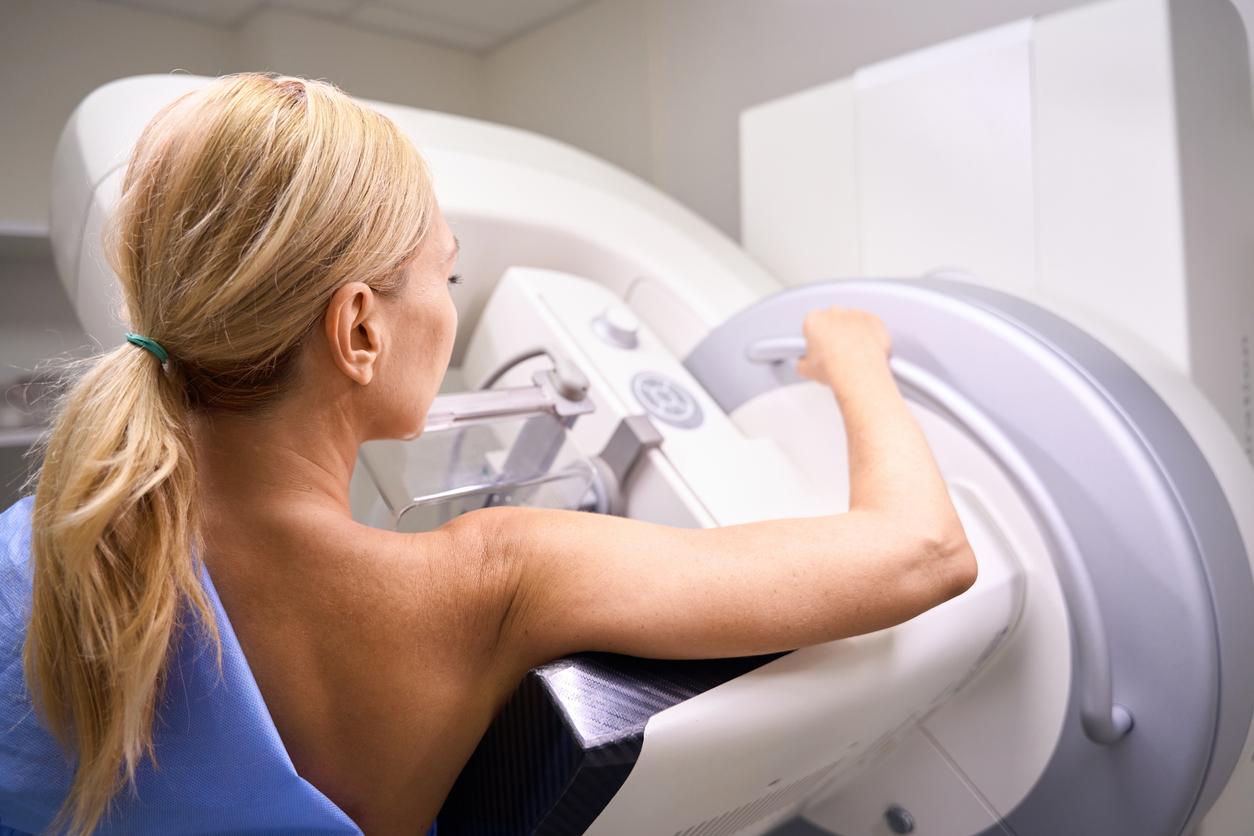
Strattice matrices, used in breast reconstruction after mastectomy, are prohibited from being placed on the market. Many side effects have been reported.

Too many side effects for the National Medicines Safety Agency (ANSM). The ban on the marketing of a medical device indicated in breast reconstruction was made public on July 6. The Strattice (Lifecell Corporation) brand biological matrix can no longer be sold or installed.
Biological matrices are used in breast reconstruction after mastectomy. It is a tissue that improves the shape and contour of the reconstructed breast. It is implanted at the same time as the prosthesis. According to the manufacturers of the different products, the medical device is associated with better healing and provides support for the implant.
Explantation cases
But the Strattice Matrix, put on 300 women, is associated with many serious side effects. In the clinical trial organized by the manufacturer, one in two women suffered from side effects. The ANSM reports cases of pain, signs of local infection or inflammation (edema, hematoma, skin necrosis) and fever. Some surgeons had to explant the medical device and / or the breast implant.
Strattice matrices will therefore have to be withdrawn from the market. In a letter sent to patients, Lifecell Corporation requests a return of any non-implanted product. Recommendations were also sent to surgeons. The ANSM asks them to inform women of the risks and possible complications and to carry out additional examinations to anticipate any reaction.
.