The Cell-Easy start-up and the Toulouse University Hospital have joined forces to carry out a study on Alzheimer’s based on stem cells obtained from cosmetic surgeons who perform liposuction.
Alzheimer’s disease is the leading cause of dementia worldwide. It represents 60 to 70% of cases. In 2015, 9.9 million people received their diagnosis, which represents a new case every three seconds according to the WHO. The disease is most often manifested by memory problems, then other brain functions are affected. Gradually, daily tasks become more and more difficult and adapting to new situations becomes almost impossible for patients. Currently, the disease is incurable and, given the number of people affected, researchers are accumulating research to at least try to slow its progression. Stem cell treatment to replace damaged cells with healthy cells is one of the therapeutic approaches that is increasingly being exploited. In France, the Toulouse University Hospital will soon start a study based on stem cells obtained from cosmetic surgeons who perform liposuction, reveals 20 minutes June 14.
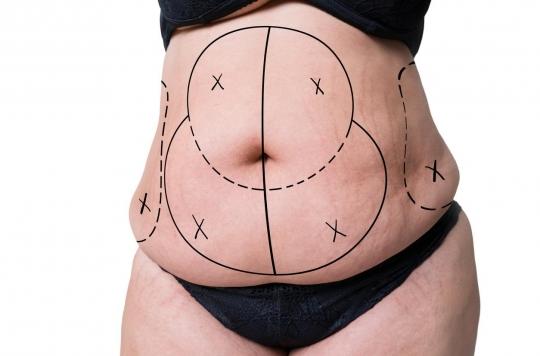
Dedicated to the large-scale production of stem cells, the Toulouse start-up Cell-Easy is the only structure of its kind in France. It recently obtained authorization to supply clinical trials with human stem cells derived from adipose tissue, which is available in almost unlimited quantities in the human body.
“Cell-Easy’s first challenge was to divide the cost of producing adipose stem cells by 10 to make this therapy accessible to as many people as possible. What we managed to do. Today we can open our pharmaceutical establishment. We are also in the process of equipping the laboratory. In about six months, production will start. The plant’s production capacity will reach 100,000 doses per year”, explains Pierre Monsan, director of Cell-Easy and founder of the French Federation of Biotechnology at The Tribune of Toulouse.
The clinical trial will start in early 2021
“We take this waste and make medicine out of it. While usually stem cells come either from umbilical cords or from painful lumbar punctures, our technology makes it possible to multiply them”, he says to 20 minutes.
The start-up has therefore just signed with the Toulouse University Hospital for a collaboration on a clinical trial on Alzheimer’s which should start in early 2021. The researchers will follow nine patients followed at the Toulouse and Montpellier University Hospitals, aged 50 to 85, newly diagnosed with the disease.
While the latter is characterized by chronic inflammation of the brain that causes protein deposits, during the study, scientists will test the anti-inflammatory effect of stem cells “and their ability to slow the progression of the disease. ”, he continues. Participants will therefore be injected with stem cells through the bloodstream. Depending on the first results, the cohort may then increase to around fifty.
Towards a biomarker for Alzheimer’s disease?
Medical research to treat Alzheimer’s is advancing rapidly. Recently, scientists discovered a promising lead for establishing a biomarker of the disease: the retina. Indeed, the upper layer of neurons in the retina of a mouse model of Alzheimer’s disease exhibits a change in their structural texture. Combined with data on changes in thickness of this layer, the new measurement could be a more easily accessible biomarker of Alzheimer’s. However, detecting the disease earlier would considerably improve the quality of life of the millions of people affected throughout the world.

.