A drug, “well tolerated” in people aged 12 and over with alopecia areata, would restore hair coverage by 80%.
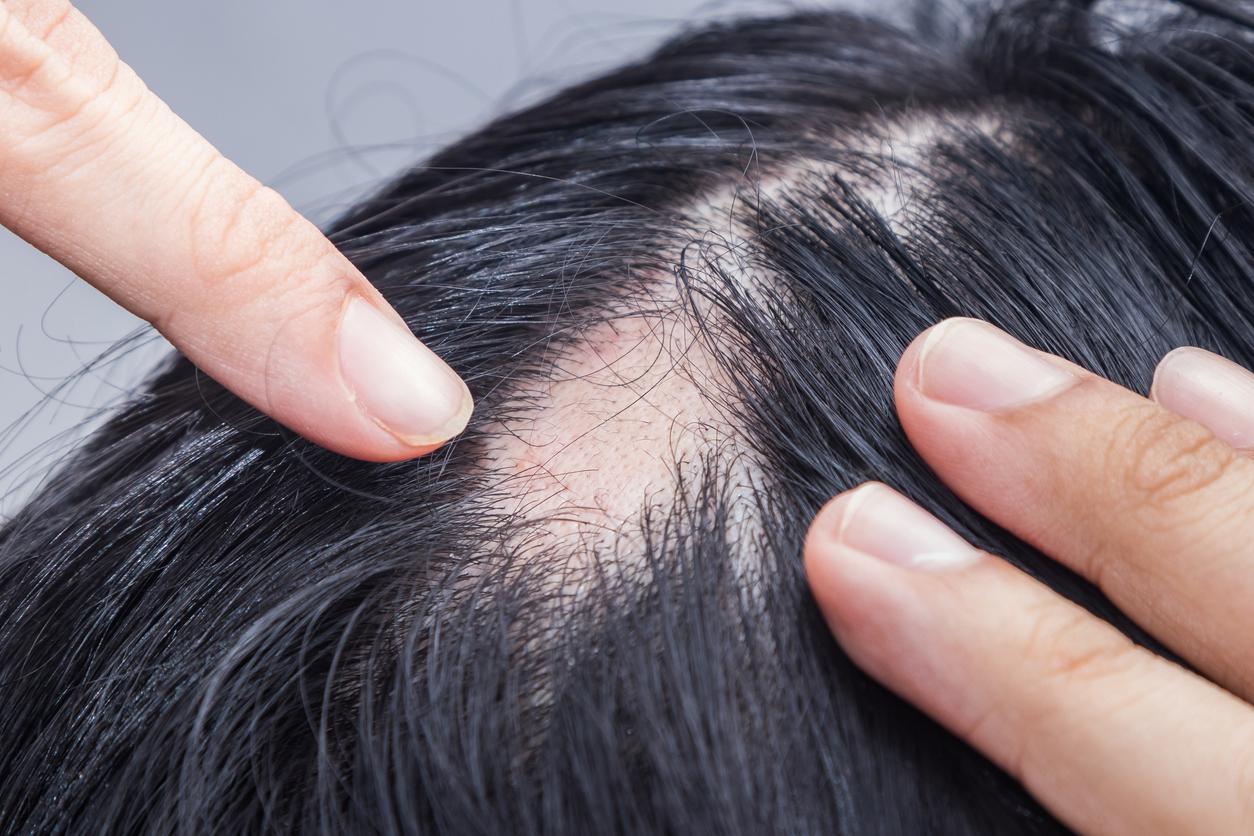
- For the first time, the Food and Drug Administration (FDA) in the United States has authorized the marketing of a drug to treat alopecia areata in patients over 12 years of age.
- The clinical trial showed that 23% of people treated had scalp hair coverage of 80% or more after taking the drug for six months.
- A few side effects such as headaches or rashes have been observed.
“Although patients can begin to develop symptoms of alopecia areata at any age, most begin to show signs in their teens, twenties, or thirties,” said Dr. Brittany Craiglow, assistant professor of dermatology at Yale School of Medicine. Until now, there was no specific treatment to cure alopecia areata, an autoimmune disease that causes non-scarring hair loss.
One tablet with a recommended dose of 50 mg per day
But, the Food and Drug Administration (FDA) recently approved a drug to fight alopecia areata from the age of 12. The treatment in question, manufactured by the Pfizer laboratory, is Ritlecitinib, but it will be sold under the name Litfulo. “The authoritative recommended dose for oral treatment is 50 mg per day”, can we read in a statement of the pharmaceutical company. In detail, the Litfulo is “a kinase inhibitor that inhibits Janus kinase 3 (JAK3) and the tyrosine kinase family of kinases expressed in hepatocellular carcinoma (TEC)”. Clearly, they target the enzymes involved in the pathogenesis of alopecia areata.
Alopecia: up to 80% recovery of scalp capillary coverage
To test the efficacy and safety of the drug, a phase 2b/3 clinical trial was conducted. A total of 718 people with hair loss of 50% or more were recruited. According to the results, published in the journal The Lancetafter six months of treatment, 23% of patients who received Litfulo had scalp hair coverage of 80% or more, compared to only 1.6% of adults who received placebo. “The drug’s efficacy and safety have been consistent in adolescents (ages 12 to 17) and adults (ages 18 and older),” the lab said.
However, as with any treatment, side effects have been reported. Based on the data, the most common side effects reported in at least 4% of participants treated with Litfulo are headache (10.8%), diarrhea (10%), acne (6.2%) , skin rashes (5.4%) and urticaria (4.6%). “Litfulo is a particularly important treatment option for young patients with severe hair loss, who are often confronted with such visible disease,” concluded Dr. Brittany Craiglow.
















