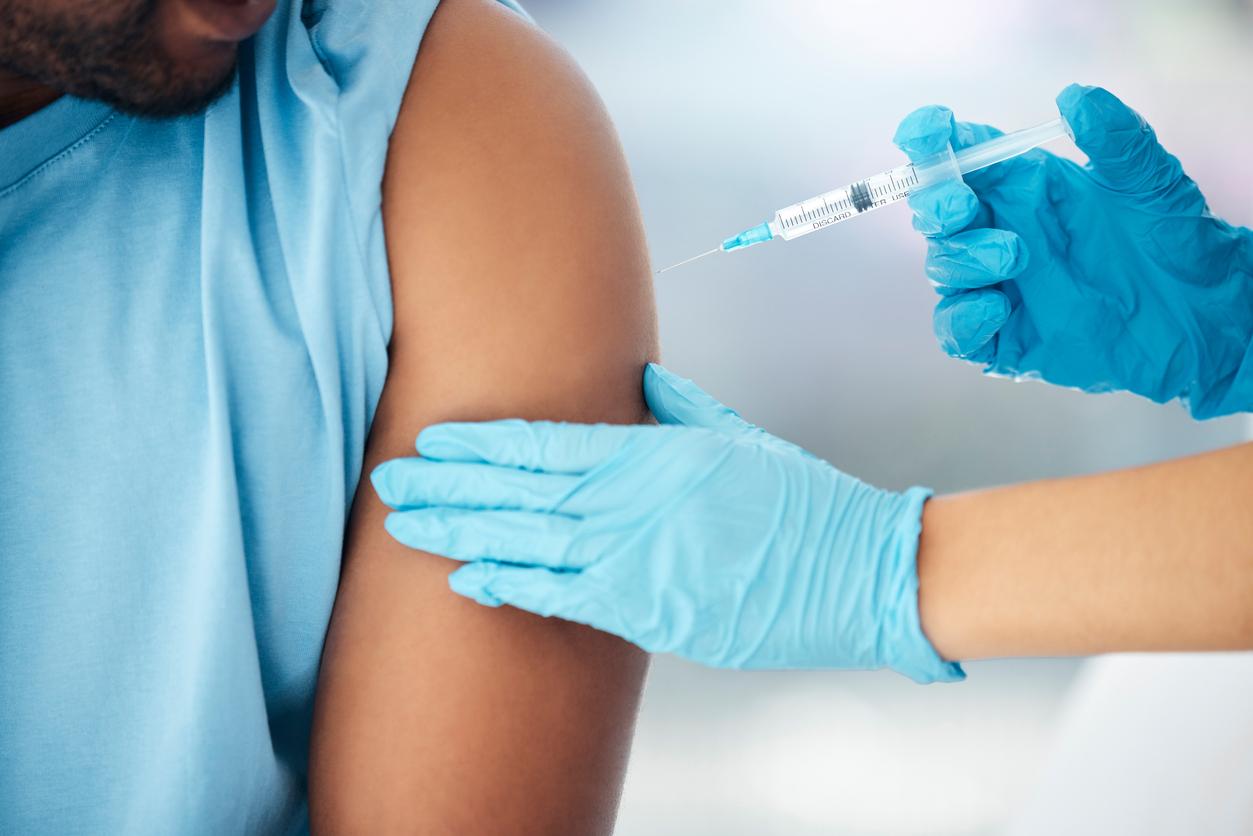
A new vaccine against Covid-19, which targets both the original strain of the virus but also the Omicron variant, is now authorized in the United Kingdom.

- Most-at-risk populations will be prioritized for the booster dose in the UK, including those aged 50 and over, pregnant women, frontline health and social care workers, immunocompromised people, etc.
- Wales has already started offering reminders to care home residents and staff, and in Northern Ireland the recall campaign is scheduled for September 19.
From this Monday, September 5, in England and Scotland, residents are invited to take a new booster dose of the vaccine against Covid-19. The first to benefit are people living in retirement homes.
Bivalent vaccine…
For this, a new version of the vaccine against Covid-19 will be used as a priority: a bivalent vaccine. This Saturday, September 3, the UK Medicines and Health Products Regulatory Agency (MHRA) announced that it approved the use of the Pfizer/BioNTech one as a booster dose for people aged 12 and over.
…which targets Omicron
Bivalent means that the vaccine targets half the original strain of the Covid-19 virus and half the Omicron variant, the most dominant in contaminations currently. The MHRA said this new version of the vaccine “complied with the safety, quality and efficiency standards of the British regulator“.
This summer, in August, the United Kingdom had already authorized a bivalent vaccine produced by Moderna and also targeting Omicron. It was the first country to do so. “I am pleased to announce that we now have a second vaccine approved for this fall’s booster program“, explained June Raine, the director of the MHRA.
Effective against BA.4 and BA.5
These new bivalent vaccines specifically target BA.1, one of the previous Omicron sub-variants (mainly BA.1 and BA.2). But they would also be effective for two more recent sub-variants of Omicron, BA.4 and BA.5, which are now in the majority.
In France
In France, for the moment, there is no marketing date for these new bivalent vaccines. But they should be used for the vaccine booster campaign which, according to theopinion of the Scientific Council published on July 19“could be open to under 60s from autumn“Currently, only those over 60 whose last injection was more than six months ago, pregnant women and anyone at risk are eligible for a fourth dose.